
Association of a genetic variant in the adenosine triphosphate transmembrane glycoprotein and risk of pancreatic cancer
Highlight box
Key findings
• The ABCB1 polymorphism (rs2032582) with increased risk of PC is associated. But this variant does not significantly associate with OS.
What is known and what is new?
• ABCB1 polymorphism is highly associated with different cancers, including PC, in women.
• This manuscript adds there is a relationship between the ABCB1 polymorphism (rs2032582) and nodal status with an increased risk of PC.
What is the implication, and what should change now?
• Further studies in larger populations and at different geographical locations are required to confirm our findings and evaluate the prognostic potentials of rs2032582 in determining the risk of PC.
Introduction
As one of the most aggressive neoplasms with a poor prognosis, pancreatic cancer (PC) is the seventh leading cause of cancer-related deaths worldwide. The incidence rate is slightly higher in men than women, as it is the twelfth most common cancer in men and eleventh in women. Moreover, age plays a role in this disease (1,2). As this disease is asymptomatic or non-specific symptoms are seen, most PC patients are diagnosed at an advanced stage (3,4). Although many efforts have been made to improve its 5-year survival rate, this remains at lower than 9% (2). The only current treatment is surgical resection followed by adjuvant chemotherapy with gemcitabine or S-1, an oral fluoropyrimidine derivative (5). KRAS, CDKN2A, TP53, and SMAD4 are the four primary driver genes for PC: one oncogene and three tumor suppressor genes. The most commonly mutated oncogene is KRAS; it encodes a small GTPase facilitating downstream signaling from growth factor receptors. The CDKN2A gene encodes the most frequently altered tumor suppressor gene which is an essential cell-cycle regulator; as in ductal adenocarcinomas, it loses function in more than 90% of cases (6). There are often somatic mutations in the TP53 tumor suppressor gene; it encodes a protein with a critical role in the cellular stress response and is mutated in a wide range of tumor types. The tumor suppressor gene SMAD4 moderates signaling downstream responsible for changing the growth factor β (TGFβ) receptor and is inactivated in about 50% of tumors (3,6).
The ABCB1 gene encodes an adenosine triphosphate (ATP) transmembrane glycoprotein, called p-glycoprotein (P-gp) or multidrug resistance 1 (MDR1), which is an efflux pump and removes toxic endogenous materials, drugs, and xenobiotics from cells (7,8). The location of the ABCB1 gene is in the 7q21.12 region, comprising a total of 209 Kb, and has 29 exons and more than 40 identified single nucleotide polymorphisms (9,10). One variant of the ABCB1 gene is the rs2032582 polymorphism (2677G>T⁄A) in exon 21. This variant can change protein function by amino acid exchange from alanine to serine or threonine (11,12), which facilitates P-gp expression (8,13). ABCB1 polymorphism is highly associated with different cancers, including PC, in women (14). Moreover, G2677T variant is notably linked to a high risk of lung cancer (10,15). Due to the involvement of the ABCB1 in the metabolism of exogenous and endogenous compounds, related polymorphism may potentially cause carcinogenesis; accordingly, we aimed to investigate the association between rs2032582 in this gene with clinical features and risk of PC.
Methods
Patient samples
In this case-control study, we have 75 PC patients and 188 age-matched healthy from Emamreza Hospital of Mashhad, Iran. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mashhad University of Medical Sciences Ethics Committee (ID: IR.MUMS.MEDICAL.REC.1400.709). The Mashhad University of Medical Sciences Ethics Committee guidelines were used to obtain informed consent from all the participants. All the samples were obtained from the patients with PC from May 2006 to August 2020 and confirmed with histological diagnosis. All the patients undergo surgical resection and survival time was calculated from the date of surgery to the date of death. Formalin-fixed, paraffin-embedded (FFPE) samples of PC tissues were obtained and used in this study. The control group was collected from the Mashhad cohort study, individuals had no diseases.
DNA extraction and genotyping
First, paraffin-embedded tissue samples were de-paraffinized by xylene and absolute ethanol solution. Genomic DNA was then extracted from FFPE tissue samples by the PZP kit, according to the provided protocol for the kit. The sample concentration and purity of DNA were assessed, then genotype analysis of rs2032582 polymorphism was carried out using Taqman®-based assay; PCR reactions were carried out in 12.5 µL total volume, using ~10 ng/µL DNA in TaqMan® Universal Master Mix with specific primer and probe (C-11711720c-30; Applied Biosystems Foster City, CA). The evaluation of the allelic content for each sample was done using the ABIPRISM-7500 tool equipped with SDS software version-2.0.
Statistical analysis
The data distribution within the subgroups was analyzed using the Kolmogorov-Smirnov tests. Normally distributed continuous data were assessed using Student’s t-tests. The comparison between the frequencies of ABCB1 gene rs2032582 polymorphisms was assessed using χ2 tests. We used independent t-test and Pearson chi-squared tests to evaluate the demographic and clinicopathological data of 252 individuals in various genotypes. The Hardy-Weinberg test was used to evaluate the genotype and allele frequency using the Pearson χ2 test. Odds ratio (OR), 95% confidence intervals (CIs), and P value were calculated to estimate the correlation of genotypes on different genetic models, using logistic regression. The data analysis was performed by SPSS-22 software with a P values less than 0.05, and all tests were two-sided.
Results
Clinicopathological characteristics of patients
The demographic, clinical, and genetic characteristics of the population are shown in Table 1. Forty-six percent of the patient samples were female, and 54% were male, with an overall mean age of 61.49±11.38 years. Moreover, 50%, 36%, and 14% of PC patients were T2, T3, and T4, respectively. Fifty-two percent of patients were in stage I–II, and 48% of patients were in stage III–IV (Table 1).
Table 1
| Characteristic | Mean ± SD or n (%) | |
|---|---|---|
| Patient | Control | |
| Age (years) | 61.04±11.99 | 57.00±9.71 |
| Sex | ||
| Female | 31 (48.0) | 90 (47.9) |
| Male | 33 (52.0) | 98 (52.1) |
| TMN classification | ||
| Stage I–II | 33 (52.0) | |
| Stage III–IV | 31 (48.0) | |
| Tumor size | ||
| T1 | 0 (0.0) | |
| T2 | 32 (50.0) | |
| T3 | 23 (36.0) | |
| T4 | 9 (14.0) | |
| Nodal status | ||
| Yes | 41 (64.0) | |
| No | 23 (36.0) | |
| Distant metastasis | ||
| Yes | 9 (14.0) | |
| No | 55 (86.0) | |
| Grade | ||
| Poor-differentiated | 0 (0.0) | |
| Moderated-differentiated | 3 (4.7) | |
| Well-differentiated | 7 (11.0) | |
| Undifferentiated | 54 (84.3) | |
Cases with unclear properties were excluded from the study. SD, standard deviation.
Association of the rs2032582 genetic variant with PC
We performed genotyping on genomic DNAs extracted from cancer patients’ tissues; hence the association between ABCB1 polymorphism (rs2032582) and susceptibility to PC was explored. As sown in the Table 2, the frequencies of AA, AC, and CC genotypes in the total population were calculated as 31.7%, 51.6%, and 16.7%, respectively which was in Hardy-Weinberg equilibrium (HWE). The frequencies of A and C alleles were 0.42 for rs2032582. The frequencies of AA, AC, and CC genotypes for rs2032582 were 29.7%, 42.2%, and 28.1%, respectively in the PC group while these frequencies in control group were 32.4%, 54.8%, and 12.8%, respectively (Table 2).
Table 2
| Gene | SNP | Control (N=188) | Case (N=64) | Total (N=252) | MAF | HWE (P value) |
|---|---|---|---|---|---|---|
| ABCB1 | rs2032582 | 0.42 | 0.37 | |||
| AA | 61 (32.4) | 19 (29.7) | 80 (31.7) | |||
| AC | 103 (54.8) | 27 (42.2) | 130 (51.6) | |||
| CC | 24 (12.8) | 18 (28.1) | 42 (16.7) |
SNP, single-nucleotide polymorphism; MAF, minor allele frequency; HWE, Hardy-Weinberg equilibrium.
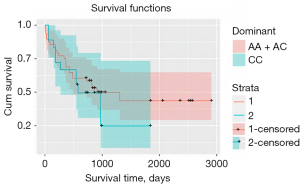
An increased risk of PC (dominant model: OR =2.67, CI =1.33–5.34, P=0.005, and recessive model: OR =1.13, CI =0.61–2.10, P=0.68) (Table 3) was observed in the individuals with an AA genotype of the ABCB1 rs2032582. We also evaluated the genotype distribution of the ABCB1 polymorphism in relation to the clinicopathological features of patients with PC using a recessive genetic model, which showed that rs2032582 has a significant correlation with nodal status (P=0.04) (Table 4). The mean range of CC genotype (28±5.8 months) was compared to AA + AC genotypes (50.8±6.7 months) and showed no significant association between the dominant genetic model and their OS (P=0.42) (Figure 1). Cox plot analysis was also performed, but like OS, this genetic model did not show a significant association with Cox regression (P=0.32).
Table 3
| Models | Genotype | Case, n (%) | Control, n (%) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Allele | A | 65 (50.8) | 225 (59.8) | – | – |
| C | 63 (49.2) | 151 (40.2) | – | ||
| Dominant | AA + AC | 46 (72.0) | 164 (87.0) | 2.67 (1.33–5.34) | 0.005 |
| CC | 18 (28.0) | 24 (13.0) | 1.00 (reference) | ||
| Recessive | AC + CC | 45 (70.0) | 127 (68.0) | 1.13 (0.61–2.10) | 0.68 |
| AA | 19 (30.0) | 156 (32.0) | 1.00 (reference) | ||
| Co-dominant | AA + CC | 37 (58.0) | 85 (45.0) | 1.66 (0.93–2.94) | 0.08 |
| AC | 27 (42.0) | 103 (55.0) | 1.00 (reference) |
OR, odds ratio; CI, confidence interval.
Table 4
| Characteristics | AA + AC (N=46) | CC (N=18) | P value |
|---|---|---|---|
| Age (years) | 47.5±11.7 | 51.0±14.4 | – |
| Sex | 0.13 | ||
| Female | 25 (54.0) | 6 (33.0) | |
| Male | 21 (46.0) | 12 (67.0) | |
| Tumor size | 0.81 | ||
| T1–T2 | 24 (52.0) | 9 (50.0) | |
| T3–T4 | 22 (48.0) | 9 (50.0) | |
| Distant metastasis | 0.6 | ||
| Yes | 6 (13.0) | 3 (17.0) | |
| No | 40 (87.0) | 15 (83.0) | |
| Nodal status | 0.04 | ||
| Yes | 26 (56.0) | 15 (83.0) | |
| No | 20 (44.0) | 3 (17.0) | |
| TMN classification | 0.81 | ||
| Stage I–II | 24 (52.0) | 9 (50.0) | |
| Stage III–IV | 22 (48.0) | 9 (50.0) | |
| Grade | 0.53 | ||
| WD, MD | 8 (17.0) | 2 (11.0) | |
| UD | 38 (83.0) | 16 (89.0) |
Data are presented as mean ± standard deviation or n (%). WD, well-differentiated; MD, moderately differentiated; UD, undifferentiated.
Discussion
The ABCB1 polymorphism is associated with a reduction in drug and metabolites transportation, so they accumulate in the extracellular environment leading to cancerous conditions. However, this polymorphism does not alter any of its encoded amino acids; it dramatically decreases mRNA expression and the stability of the protein and may be associated with tumorigenesis (16). Our results showed that PC is more common in male than in female. Our data are in line with a previous study in Tehran population. Sheikh et al. revealed that most patients were male (61.1%) with lower overall survival (OS) (17).
An important recent finding has shown the significant association between a genetic variant (rs2032582) and the risk of PC. Individuals with an AA genotype are more susceptible to PC. There is a piece of emerging evidence supporting the notion that the rs2032582 genetic variant plays a role in the development of several malignancies (18), such as lung cancer (15) and colorectal cancer (10,14). Consistent with our results, a recent study by ShahidSales et al. in 2020 on 88 breast cancer and 200 healthy individuals reported no statistically significant association between CYP1B1/rs1056836 and the type and the risk of breast cancer. At the same time, ABCB1/rs2032582 was potentially associated with breast cancer tumor size (P<0.05) (10). Regarding the association between the ABCB1 gene rs1045642 polymorphism, a meta-analysis consisting of 3,175 colorectal cancer patients and healthy controls in 2012 was undertaken. The result showed that neither ABCB1 rs2032582 nor ABCB1 rs3789243 were associated with colorectal cancer risk (P=0.03). Neither ABCB1 rs2032582 nor ABCB1 rs3789243 were associated with colorectal cancer risk. He et al. found an increased frequency of alleles (rs2032582G/rs1045642C) in Caucasian CRC patients (P=0.02) (8). In a study done by Panczyk et al. in the Netherlands, 95 patients with colorectal cancer and 95 healthy blood samples, every 3 SNP were tested (ABCB11236C>T, ABCB12677G>T/A, and ABCB13435C>T). Haplotypes were significantly distributed among colorectal patients and the healthy population (P=0.03). Differences in haplotype distributions between colorectal cancer patients and healthy populations suggested that other potential SNPs, especially in the regulatory region of the ABCB1 gene, may influence P-glycoprotein expression and function (19). The association of ABCB1 3435>T polymorphism and treatment outcomes in advanced gastric cancer patients was observed in a study by Chang et al. In this study, 43 gastric cancer patients were treated with paclitaxel-based chemotherapy and a control group of 11 healthy volunteers (20). In 2019, Zhao showed the effects of OPRM1 and ABCB1 gene polymorphisms (rs2032582 and rs1128503) on the analgesic effect and dose of sufentanil after thoracoscopic-assisted radical resection of lung cancer. In this study, 225 patients were included (132 men and 93 women). The results showed that the sufentanil doses at T1 (doses and side effects of sufentanil consumed 6 h), T2 (24 h), and T3 (48 h) were significantly higher in radical-operation lung cancer patients with mutant homozygous rs2032582 and rs1128503 loci in the ABCB1 gene. Patients at T1, T2, and T3 took higher doses of sufentanil in comparison to patients without mutations, with a statistically significant difference (P<0.05). In the current study, there was no significant difference between rs1045642 and sufentanil consumption (P>0.05), while previous investigation showed a positive association between the variation and PC (21). Pang et al., in 2014, investigated the potential relationship between the progression of PC and chemical resistance. In this study, 4 SNPs in different genes, including ABCB1, and the expression of 3 transporters were investigated in healthy and cancerous samples (22).
Conclusions
Our outcomes show a relationship between the ABCB1 polymorphism (rs2032582) and nodal status and an increased risk of PC. Further studies in larger populations and at different geographical locations are required to confirm our findings and evaluate the prognostic potentials of rs2032582 in determining the risk of PC.
Acknowledgments
Funding: This research was supported by Mashhad University of Medical Sciences (No. 981618).
Footnote
Data Sharing Statement: Available at https://apc.amegroups.org/article/view/10.21037/apc-22-7/dss
Peer Review File: Available at https://apc.amegroups.org/article/view/10.21037/apc-22-7/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.org/article/view/10.21037/apc-22-7/coif). All authors report that this research was supported by Mashhad University of Medical Sciences (No. 981618). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Mashhad University of Medical Sciences Ethics Committee (ID: IR.MUMS.MEDICAL.REC.1400.709). The Mashhad University of Medical Sciences Ethics Committee guidelines were used to obtain informed consent from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khalaf N, El-Serag HB, Abrams HR, et al. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin Gastroenterol Hepatol 2021;19:876-84. [Crossref] [PubMed]
- Qin C, Yang G, Yang J, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer 2020;19:50. [Crossref] [PubMed]
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol 2020;17:527-40. [Crossref] [PubMed]
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020;395:2008-20. [Crossref] [PubMed]
- Wood LD, Hruban RH. Pathology and molecular genetics of pancreatic neoplasms. Cancer J 2012;18:492-501. [Crossref] [PubMed]
- Hartmann G, Kim H, Piquette-Miller M. Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int Immunopharmacol 2001;1:189-99. [Crossref] [PubMed]
- He T, Mo A, Zhang K, et al. ABCB1/MDR1 gene polymorphism and colorectal cancer risk: a meta-analysis of case-control studies. Colorectal Dis 2013;15:12-8. [Crossref] [PubMed]
- Vencatto RW, Ramalho S, Marson FAL, et al. ABCB1 variants (C1236T, rs1128503 and G2677T/A, rs2032582) do not show an association with recurrence and survival in patients with breast cancer undergoing anthracycline-based chemotherapy. Meta Gene 2019;21:100596. [Crossref]
- ShahidSales S. The association between genetic variants in the genes for cytochrome P450 B1 and ATP-binding cassette transporter genes and breast cancer risk. Mol Biol Rep 2020;47:6009-14. [Crossref] [PubMed]
- Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A 2000;97:3473-8. [Crossref] [PubMed]
- Cascorbi I, Gerloff T, Johne A, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther 2001;69:169-74. Erratum in: Clin Pharmacol Ther 2004;75:124. [Crossref] [PubMed]
- Riera P, Artigas-Baleri A, Salazar J, et al. ABCB1 Genetic Variants as Predictors of Irinotecan-Induced Severe Gastrointestinal Toxicity in Metastatic Colorectal Cancer Patients. Front Pharmacol 2020;11:973. [Crossref] [PubMed]
- Sainz J, Rudolph A, Hein R, et al. Association of genetic polymorphisms in ESR2, HSD17B1, ABCB1, and SHBG genes with colorectal cancer risk. Endocr Relat Cancer 2011;18:265-76. [Crossref] [PubMed]
- Gervasini G, Carrillo JA, Garcia M, et al. Adenosine triphosphate-binding cassette B1 (ABCB1) (multidrug resistance 1) G2677T/A gene polymorphism is associated with high risk of lung cancer. Cancer 2006;107:2850-7. [Crossref] [PubMed]
- Tamura M, Kondo M, Horio M, et al. Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity. Nagoya J Med Sci 2012;74:133-40. [PubMed]
- Sheikh M, Masoudi S, Bakhshandeh R, et al. Survival features, prognostic factors, and determinants of diagnosis and treatment among Iranian patients with pancreatic cancer, a prospective study. PLoS One 2020;15:e0243511. [Crossref] [PubMed]
- Beuten J, Gelfond JA, Byrne JJ, et al. CYP1B1 variants are associated with prostate cancer in non-Hispanic and Hispanic Caucasians. Carcinogenesis 2008;29:1751-7. [Crossref] [PubMed]
- Panczyk M, Balcerczak E, Piaskowski S, et al. ABCB1 gene polymorphisms and haplotype analysis in colorectal cancer. Int J Colorectal Dis 2009;24:895-905. [Crossref] [PubMed]
- Chang H, Rha SY, Jeung HC, et al. Association of the ABCB1 3435C>T polymorphism and treatment outcomes in advanced gastric cancer patients treated with paclitaxel-based chemotherapy. Oncol Rep 2010;23:271-8. [PubMed]
- Zhao Z, Lv B, Zhao X, et al. Effects of OPRM1 and ABCB1 gene polymorphisms on the analgesic effect and dose of sufentanil after thoracoscopic-assisted radical resection of lung cancer. Biosci Rep 2019;39:BSR20181211. [Crossref] [PubMed]
- Pang L, Word B, Xu J, et al. ATP-Binding Cassette Genes Genotype and Expression: A Potential Association with Pancreatic Cancer Development and Chemoresistance? Gastroenterol Res Pract 2014;2014:414931. [Crossref] [PubMed]
Cite this article as: Khojasteh-Leylakoohi F, Khalili-Tanha G, Sardarzadeh N, Khalili-Tanha N, Dashtiahangar M, Mehrabadi S, Asadnia A, Avan A, Amoueian S, Hassanian SM, Esmaily H, Khazaei M, Ferns GA, Aliakbarian M, Khooei A. Association of a genetic variant in the adenosine triphosphate transmembrane glycoprotein and risk of pancreatic cancer. Ann Pancreat Cancer 2023;6:6.


