
Neoadjuvant therapy in upfront resectable pancreatic cancer: current evidence and future considerations
Introduction
Pancreatic cancer remains the fourth leading cause of cancer death in both men and women in the U.S. with a dismal 5-year survival rate of 8% across all stages (1). Pancreatic ductal adenocarcinoma (PDAC) comprises approximately 80% of all pancreatic cancers, and surgical resection remains the only potential cure for this lethal malignancy in the <20% of patients that present with resectable disease (2). The median survival is 17–27 months in those with resected pancreatic cancer and the current standard of care following surgery in this population is adjuvant chemotherapy or chemoradiation (3).
However, disease recurrence occurs in 66–92% of patients within 2 years of resection with local recurrence rates of 35–60% and systemic recurrence rates as high as 80–90% (3). Patients who undergo successful surgical resection with microscopically negative margins (R0) and node-negative disease have historically shared a more favorable prognosis than those with microscopically (R1) or macroscopically (R2) positive margins and node-positive disease (4). Accordingly, therapies that can optimize R0 resection rates, decrease local recurrence, and reduce systemic recurrence represent potential strategies to improve outcomes in the subset of pancreatic cancer patients with resectable disease that are most likely to survive.
Neoadjuvant or preoperative therapy has recently been the subject of growing clinical investigation in the treatment paradigm of upfront resectable pancreatic cancer. In this review, we highlight recent findings from prospective clinical trials investigating the efficacy of neoadjuvant therapy in radiographically resectable pancreatic cancer. We provide a critical appraisal of the current state of neoadjuvant therapy in the management of initially resectable pancreatic cancer. We also discuss the potential advantages and disadvantages to a neoadjuvant or upfront surgery approach and review ongoing clinical trials seeking to provide further clarity on the utility of neoadjuvant therapy.
Search criteria
A literature search using the keywords “neoadjuvant” and “pancreatic cancer” was conducted through MEDLINE to include published studies up to March 10, 2018. The search returned a total of 1,368 hits. Limiting the search to clinical studies of prospective design, English language, and involving neoadjuvant therapy in resectable pancreatic cancer, a final 29 studies were included in this review (Tables 1,2).
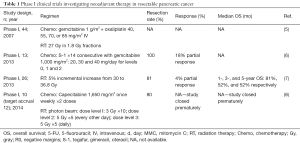
Full table
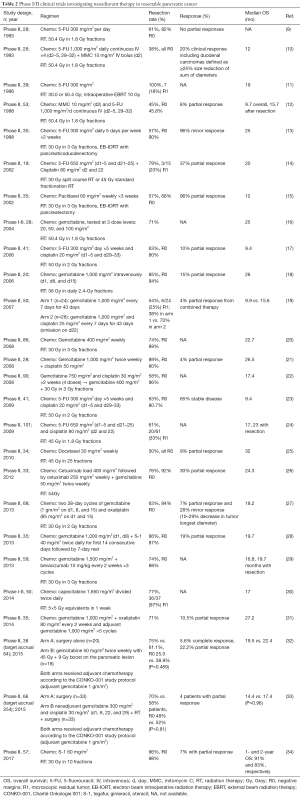
Full table
Clinical trials of neoadjuvant therapy in resectable pancreatic cancer
Phase I trials
Preoperative therapy (primarily radiation therapy or RT-based modalities) in pancreatic cancer was first investigated more than 2 decades ago with the intent to improve rates of local control, resectability, and distant metastases (35,36). A phase I study enrolled 44 patients with 12 patients with resectable pancreatic adenocarcinoma to determine the dose-limiting toxicity (DLT) of biweekly oxaliplatin and gemcitabine with concurrent RT (5). Ten DLTs were observed including grade 3 platelet disorder (n=4), decline in functional status (n=2), gastrointestinal (GI) bleed (n=2), and GI toxicity (n=2). The study concluded that biweekly oxaliplatin 85 mg/m2 with full-dose gemcitabine and concurrent RT is well tolerated. One phase I study involving 11 patients evaluated the DLTs and MTD of neoadjuvant gemcitabine plus oral S-1 in resectable pancreatic adenocarcinoma (6). The authors observed a 100% rate of resectability with 22% of patients demonstrating a partial response (PR). However, the treatment regimen was not well-tolerated due to grade ≥3 toxicities including liver dysfunction, neutropenia, and anorexia.
A separate phase I study involving 26 patients sought to determine the recommended dose of carbon-ion radiotherapy needed to reduce the risk of postoperative local recurrence in patients with resectable pancreatic adenocarcinoma (9). All patients completed the scheduled treatment course without treatment breaks, and no DLT was observed (Table 1). Another phase I study investigated the feasibility and tolerability of neoadjuvant short course radiotherapy (SC-CRT) delivered with photon RT concurrent with capecitabine in patients with resectable pancreatic adenocarcinoma (10). The intended accrual for the study was 12 patients; however, the study was closed prematurely due to unexpected intraoperative complications. Specifically, the main surgical complication encountered was increased intraoperative radiation fibrosis reported by surgeons, which ultimately translated to increased mean operative time. No patients had surgery delayed because of acute treatment-related toxicities.
Phase II trials
A number of phase II trials have been conducted over the past 2 decades to further explore the potential role of neoadjuvant chemoradiation in patients with pancreatic adenocarcinoma (Table 2). Among the first prospective clinical trials incorporating chemotherapy and RT was a phase II study involving 26 patients with biopsy-proven localized pancreatic cancer treated with neoadjuvant RT concurrent with 5-flourouracil (5-FU) and mitomycin C (within 24 hours of starting RT) that produced a 38% resectability rate (10). Including the cohort of duodenal cancers, all but 2 patients were able to complete the planned preoperative chemoRT (1 biliary catheter-related sepsis and 1 myelosuppression). A number of other phase II studies have assessed the safety and efficacy of 5-FU-based chemoradiotherapy to treat patients with resectable pancreatic adenocarcinoma (9-14,17,23,24). Resectability rates varied from 45–100% with rates of R0 resections ranging from 45.8–100%. No complete responses (CRs) were observed. The median overall survival (OS) ranged from 9.4–24 months. Overall, neoadjuvant therapies were well tolerated with no delay in surgery from treatment-related toxicities reported.
Gemcitabine is well-known to be a potent radiosensitizer that has shown clinical superiority compared to 5-FU in the treatment of advanced pancreatic cancer (Table 2). A number of phase II studies have sought to evaluate its potential role in neoadjuvant therapy for patients with resectable pancreatic adenocarcinoma (16,18). One study was stopped prematurely for poor accrual rate (32). Rates of resection varied from 61–85% with R0 resection rates varying from 40–90% (Table 2). Other studies have further expanded the use of gemcitabine with the addition of cisplatin because the two agents have differing mechanisms of action and lack of cross-resistance (19-22,33). Resectability rates ranged from 58–89% with R0 resection rates varying from 52–96% (Table 2). One randomized-controlled trial had promising results demonstrating improved resection rates and encouraging survival with use of combination therapy (19). However, one study suggested that the combination did not enhance survival beyond that achieved with neoadjuvant gemcitabine-based chemoradiotherapy alone (22). Another study has suggested there was an improvement in quality-of-life and improvement in nutritional status with combination gemcitabine and cisplatin (21). A separate randomized-control trial reportedly ended prematurely for slow accrual (33). In that study, combination therapy was well-tolerated, however no CRs were observed. Other combination therapies with gemcitabine include oxaliplatin and S-1 with trials demonstrating promising R0 resection rates (27,28,31).
Other promising agents have also been explored in phase II trials (Table 2). Two taxane-based regimens were studied and demonstrated resection rates ranging from 50–57% with R0 resection rates varying from 68–100% (15,25). Median OS ranged from 12–32 months. Overall the regimens were well-tolerated. A study evaluating the use of cetuximab given prevalence of EGFR overexpression in pancreatic adenocarcinomas was able to achieve a 76% rate of resection (26). Another study explored the use of bevacizumab given its potential synergism with gemcitabine (29). While the regimen was well-tolerated and achieved a rate of surgical resection of 74%, the rate of complete pathologic response was not superior to other regimens.
Given concern for the metastatic propensity of pancreatic adenocarcinoma, there has been a strong interest to reduce the duration of RT. A phase I-II trial utilized a 2-week proton-based radiation with capecitabine and was able to demonstrate the regimen was well tolerated with a 4.1% grade 3 toxicity rate (30). Another phase II trial utilizing hypo-fractionated RT with S-1 demonstrated a 96% rate of resectability with the authors attributing the high resection rate to the short 2-week treatment duration (34).
Meta-analyses of clinical studies on neoadjuvant therapies in resectable pancreatic cancer
Given the growing body of evidence concerning neoadjuvant therapy in resectable pancreatic cancer, several meta-analyses have recently been conducted to further clarify on the benefit of this approach. Gillen et al. identified 111 retrospective and prospective studies involving 4,394 patients between 1980 to 2009 to assess tumor response, resection rates, survival rate, and toxicities in resectable, borderline, and unresectable pancreatic cancer (37). The most common chemotherapeutic regimens included gemcitabine, 5-FU, platinum-based regimens, and mitomycin C within the studies identified. For patients with resectable disease, a resectability rate of 73.6% [95% confidence interval (CI): 65.9–80.6%] was achieved. The authors observed CR and PR rates of 3.6% (95% CI: 2–5.5%) and 30.6% (95% CI: 20.7–41.4%), respectively, and the median OS after resection was 23.3 (range, 12–54) months. Because the rates of resection in this analysis were similar to the rates published in the literature of patients who did not receive neoadjuvant therapy, the authors questioned the utility of neoadjuvant therapy in patients with resectable pancreatic cancer.
Assifi et al. identified 14 prospective phase II trials that were predominantly single-arm studies comprising 536 patients and evaluated the role of neoadjuvant chemoradiotherapy in both resectable, borderline, and unresectable pancreatic cancer (38). In patients with resectable disease, resectability after chemoradiation was 65.8% (95% CI: 55.4–75.6%) with an 85.1% R0 resection rate. There was a large proportion of SD (73.9%, 95% CI: 63.2–83.3%) compared to PRs (9.5%, 95% CI: 2.9%–19.4%). In addition, the median OS was 23.0 months (range, 11.7–34 months). Given these results, the authors concluded that the role and impact of neoadjuvant therapy within resectable disease remains unclear given similar outcomes reported in the literature in patients who do not receive neoadjuvant therapy.
Andriulli et al. identified 20 prospective phase I–II studies involving 707 patients utilizing neoadjuvant gemcitabine-based therapy with or without radiation in patients with localized pancreatic cancer (39). Including both resectable and unresectable cases, the CR and PR rates were 12% (95% CI: 4–23%) and 27% (95% CI: 18–38%), respectively. In patients with resectable disease, a resectability rate of 91% (95% CI: 83–97%) was achieved with 89% R0 resections (95% CI: 83–94%). The median OS was 18.7 months (95% CI: 9–32%). However, with a treatment-related grade 3–4 toxicity of 29% (95% CI: 14–47%) and lingering questions regarding whether survival is enhanced by neoadjuvant therapy, the authors concluded there is marginal support for the benefits of neoadjuvant therapies in patients with potentially resectable disease.
D’Angelo et al. identified 16 randomized controlled trials between 1985 and 2015 focusing on adjuvant and neoadjuvant therapy to evaluate OS and protocol achievement in resectable pancreatic cancer (40). In the neoadjuvant setting, the OS varied between 9.9 and 19.4 months, 12.5–29.8 months with adjuvant therapy, and 11 and 20.2 months with surgery only. Protocol achievement ranged between 18.18% and 70.00% for patient treated with neoadjuvant therapy. Given these findings, the authors believe adjuvant therapy should still remain the standard of care. In addition, the authors advocate for studies to determine whether neoadjuvant therapy can enhance patient outcomes in patients who adjuvant therapy instead of designing trials to compare neoadjuvant vs. adjuvant therapy. Verma et al. included 30 prospective phase II trials to assess the postoperative morbidity and mortality in patients with pancreatic cancer who received neoadjuvant chemotherapy or chemoradiotherapy (41). Common postoperative complications in patients with resectable or borderline disease who received neoadjuvant chemoradiotherapy included delayed gastric emptying (6–15%), pancreatic leaks (3–7%), sepsis (3–19%), hemorrhage (2–13%), and fistula formation (2–3%). 9/13 studies demonstrated a mortality of 4%. Patients who received neoadjuvant chemotherapy demonstrated comparable complications with pancreatic leaks (3–11%), fistula rates (3–4%), sepsis (3–7%) and mortality (0–4%). The rates of complications were similar to patients who received surgery only. Hence, the authors concluded that neoadjuvant chemotherapy or chemoradiotherapy is safe based on post-operative outcomes.
D’Angelo et al. included 12 prospective studies from 2008 to 2015 comprising 624 patients with resectable, borderline, and locally advanced disease to evaluate the rate of protocol achievement and OS (42). The most common chemotherapeutic agent used was gemcitabine. The authors reported a pooled protocol achievement rate of 65% (95% CI: 62–67%) with a 94% R0 resection rate. The OS from patients with resectable disease who eventually received resection was not significantly different in comparison to the survival rate within the total cohort [20.87 (95% CI: 17.97–23.82 months) vs. 22.78 months (95% CI: 20.42–25.16 months)]. Given the lack of strong evidence, the authors believe that further studies with randomized trials to clarify the benefits of neoadjuvant therapy are needed. While previous meta-analyses are plagued by heterogeneity in anatomic definitions for PDACs, there have been significant advances to standardize the definitions with release of expert consensus criteria in 2009. In one meta-analysis, Dhir et al. evaluated 96 retrospective and prospective phase I–II studies from 2009 involving 5,520 patients with resectable (n=1,056), borderline (n=935), and unresectable (n=1,840) pancreatic cancer treated with neoadjuvant therapy (43). While there have been concerns in regards to disease progression during neoadjuvant therapy, the incidence of progression was rare (11%). In patients with resectable disease, the authors reported a resectability rate of 76% with a R0 resection rate of 63%. A PR rate of 11% was observed. In patients with resectable disease who underwent surgery, the median OS was 30 months. Grade ≥3 toxicity was observed in 36% of the patients (95% CI: 27–45%) with 91% able to complete planned therapy. In short, the authors concluded these results demonstrate neoadjuvant therapy as a potential avenue for treatment in patients with resectable PDAC.
Zhan et al. identified 39 prospective studies involving 1,458 patients with 14 studies specifically focusing on evaluating the safety and efficacy of neoadjuvant therapy in patients with resectable pancreatic disease (44). The authors observed a resectability rate of 73% with a R0 resection rate of 84.2%. The CR and PR rate was 1.8% and 14.6%, respectively. OS was 17.76 months without resection and 24.24 months with resection. The incidence of grade ≥3 toxicities was 11.3%. Given these findings, neoadjuvant therapy has yet to demonstrate clinical superiority. In addition, considering the risk of disease progression, the authors concluded that neoadjuvant chemotherapy may not be beneficial in patients with resectable disease.
Discussion
The incorporation of systemic therapies ± RT with surgery have afforded improvements in survival in patients with resectable pancreatic cancer (3,4). However, there is a growing debate as to whether neoadjuvant or adjuvant therapy represents the appropriate management approach to optimize survival in resectable disease—the only potentially curable population of pancreatic cancer patients. Some have criticized that not all patients who undergo potentially curative resection receive adjuvant therapy (contemporary estimates of 40–50% of patients receive adjuvant therapy after surgery) (45). Furthermore, postoperative complications and mortality can impede delivery of adjuvant therapy. R0 resections confer more favorable prognosis than non-R0 resections; by virtue of the limits of preoperative imaging in detecting microscopic disease, upfront surgical approaches prohibit the ability to achieve meaningful decreases in tumor reduction or identify patients with aggressive disease who would otherwise not benefit from surgery. Lastly, pancreatic surgery has been shown to be immunosuppressive and may promote metastases that may otherwise be reduced by preoperative therapy.
Proponents of neoadjuvant therapy in upfront resectable pancreatic cancer have argued that this approach: (I) increases the chances for R0 resection; (II) increases the likelihood to complete multimodality therapy; (III) minimizes pancreatic leak; (IV) increases the efficacy of RT; (V) improves cost-effectiveness; and (VI) identifies poor candidates for surgery including either those with poor performance status, aggressive tumor biology, or unanticipated metastases (46). There is a growing body of evidence to support the feasibility of neoadjuvant therapy in radiographically resectable pancreatic cancer (Tables 1,2). Many studies have demonstrated that preoperative therapy produces survival durations for resected patients that are often equivalent, if not superior, to outcomes with upfront surgery and adjuvant therapy (45). Other studies, however, have shown mixed results with respect to a neoadjuvant approach. Furthermore, neoadjuvant therapy does carry its own set of risks including disease progression during preoperative therapy for disease that was initially resectable and potentially curable and the lack of large, prospective randomized phase III trials with level 1 evidence to support this approach over adjuvant therapy (4,46).
Several major guidelines currently recognize neoadjuvant therapy as an option within the treatment paradigm for resectable pancreatic cancer (47,48). In short, neoadjuvant therapy can be considered in select patients with technically resectable disease and high-risk features including: markedly elevated CA 19-9 levels or radiographic findings suspicious but not diagnostic of metastatic disease, poor performance status or comorbidities rendering the patient not fit for major abdominal surgery, large primary tumors, large regional lymph nodes, excessive weight loss, or extreme pain. The American Society of Clinical Oncology (ASCO) posits that neoadjuvant therapy can be offered as an alternative strategy for patients who meet criteria for upfront resectable disease (47). However, the National Comprehensive Cancer Network (NCCN) does not recommend neoadjuvant therapy for clearly resectable pancreatic cancer without high-risk features as such an approach in this setting should take place in the context of a clinical trial (48).
These recommendations have been put forth with the understanding that although benefits may outweigh harms for a neoadjuvant approach, the quality of evidence supporting this strategy is low. Beyond the need for high-level evidence from larger, prospective randomized clinical trials supporting a neoadjuvant approach, there still remains several questions to this treatment strategy in resectable pancreatic cancer. For one, it is unclear which modality represents the optimal strategy in a neoadjuvant approach: chemotherapy, RT, or combined modality? Furthermore, in patients who have received preoperative therapy for localized pancreatic cancer, what is the role of adjuvant therapy (49,50)? With the understanding that there are no data from randomized clinical trials to answer this question, major guidelines recommend a total of 6 months of adjuvant therapy (including the duration of the neoadjuvant regimen) as extrapolated from adjuvant therapy trials (47). This decision should be based on multidisciplinary review and the choice of adjuvant regimen should be based on response to the neoadjuvant regimen and anticipated tolerability; adjuvant therapy should only be administered in those without evidence of recurrent or distant disease and who have recovered from surgery (ideally within 4–8 weeks) (48).
Additionally, are there evidence-based criteria or definitive predictors to guide selection of candidates who would benefit most from neoadjuvant therapy? One phase I/II trial associated KRASG12D mutation status and high CXCR7, CEA, CA 19-9, and HGF levels to worse survival in patients treated with neoadjuvant chemoradiation (30). A separate group identified a 6-gene signature that predicts survival in localized PDAC and could potentially be used to select candidates for neoadjuvant therapy (51). Higher levels of tumor-infiltrating lymphocytes and a higher ratio of CD8/FOXP3 lymphocytes were associated with improved OS in PDAC patients treated with neoadjuvant therapy (52). Lastly, hENT1, TS/DPD, EGFR, SPARC, and SMAD4 are among the growing list of potential biomarkers that may guide selection of patients with resectable pancreatic cancer who would benefit most from neoadjuvant therapy (46). Reassuringly, there are several ongoing phase II and III trials that may potentially address several questions that remain unanswered in the neoadjuvant treatment of resectable pancreatic cancer (Table 3). Results from these studies are eagerly awaited to see if neoadjuvant therapy is truly primed to establish itself as an integral component of the treatment paradigm for localized resectable pancreatic cancer.
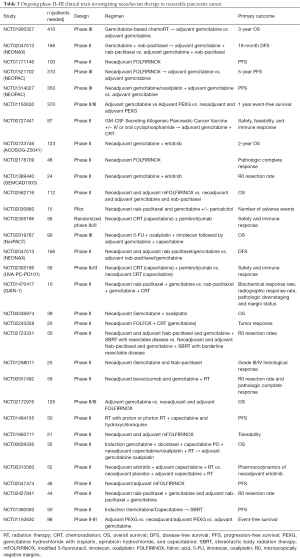
Full table
Conclusions
Evidence is accumulating to support the feasibility and efficacy of neoadjuvant therapy in radiographically resectable pancreatic cancer. Several major national guidelines now recognize neoadjuvant therapy as an option in the treatment of resectable pancreatic cancer. Nevertheless, high-level evidence is lacking to guide the selection of patients who would benefit most from neoadjuvant therapy, choice of optimal modality in a neoadjuvant strategy, and need for adjuvant therapy following neoadjuvant therapy. Results from ongoing, randomized prospective clinical trials may provide further clarity to several questions remaining unanswered in the neoadjuvant treatment of resectable pancreatic cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [Crossref] [PubMed]
- Gong J, Tuli R, Shinde A, et al. Meta-analyses of treatment standards for pancreatic cancer. Mol Clin Oncol 2016;4:315-25. [Crossref] [PubMed]
- Abbott DE, Baker MS, Talamonti MS. Neoadjuvant therapy for pancreatic cancer: a current review. J Surg Oncol 2010;101:315-20. [Crossref] [PubMed]
- Desai SP, Ben-Josef E, Normolle DP, et al. Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J Clin Oncol 2007;25:4587-92. [Crossref] [PubMed]
- Tajima H, Kitagawa H, Tsukada T, et al. A phase I study of neoadjuvant chemotherapy with gemcitabine plus oral S-1 for resectable pancreatic cancer. Mol Clin Oncol 2013;1:768-72. [Crossref] [PubMed]
- Shinoto M, Yamada S, Yasuda S, et al. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer 2013;119:45-51. [Crossref] [PubMed]
- Wo JY, Mamon HJ, Ferrone CR, et al. Phase I study of neoadjuvant accelerated short course radiation therapy with photons and capecitabine for resectable pancreatic cancer. Radiother Oncol 2014;110:160-4. [Crossref] [PubMed]
- Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992;127:1335-9. [Crossref] [PubMed]
- Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A phase II study. Cancer 1993;72:2124-33. [Crossref] [PubMed]
- Staley CA, Lee JE, Cleary KR, et al. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg 1996;171:118-24. [Crossref] [PubMed]
- Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol 1998;16:317-23. [Crossref] [PubMed]
- Pisters PW, Abbruzzese JL, Janjan NA, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol 1998;16:3843-50. [Crossref] [PubMed]
- Moutardier V, Giovannini M, Lelong B, et al. A phase II single institutional experience with preoperative radiochemotherapy in pancreatic adenocarcinoma. Eur J Surg Oncol 2002;28:531-9. [Crossref] [PubMed]
- Pisters PW, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol 2002;20:2537-44. [Crossref] [PubMed]
- Joensuu TK, Kiviluoto T, Kärkkäinen P, et al. Phase I-II trial of twice-weekly gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy with extended lymphadenectomy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;60:444-52. [Crossref] [PubMed]
- Mornex F, Girard N, Scoazec JY, et al. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: The French SFRO-FFCD 97-04 phase II trial. Int J Radiat Oncol Biol Phys 2006;65:1471-8. [Crossref] [PubMed]
- Talamonti MS, Small W, Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol 2006;13:150-8. [Crossref] [PubMed]
- Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol 2007;14:2088-96. [Crossref] [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [Crossref] [PubMed]
- Heinrich S, Pestalozzi BC, Schäfer M, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:2526-31. [Crossref] [PubMed]
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [Crossref] [PubMed]
- Le Scodan R, Mornex F, Girard N, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol 2009;20:1387-96. [Crossref] [PubMed]
- Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a ten-year single institution experience. Oncology 2009;76:413-9. [Crossref] [PubMed]
- Turrini O, Ychou M, Moureau-Zabotto L, et al. Neoadjuvant docetaxel-based chemoradiation for resectable adenocarcinoma of the pancreas: New neoadjuvant regimen was safe and provided an interesting pathologic response. Eur J Surg Oncol 2010;36:987-92. [Crossref] [PubMed]
- Pipas JM, Zaki BI, McGowan MM, et al. Neoadjuvant cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with pancreatic adenocarcinoma. Ann Oncol 2012;23:2820-7. [Crossref] [PubMed]
- Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119:2692-700. [Crossref] [PubMed]
- Motoi F, Ishida K, Fujishima F, et al. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreatic ductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol 2013;20:3794-801. [Crossref] [PubMed]
- Van Buren G, Ramanathan RK, Krasinskas AM, et al. Phase II study of induction fixed-dose rate gemcitabine and bevacizumab followed by 30 Gy radiotherapy as preoperative treatment for potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol 2013;20:3787-93. [Crossref] [PubMed]
- Hong TS, Ryan DP, Borger DR, et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int J Radiat Oncol Biol Phys 2014;89:830-8. [Crossref] [PubMed]
- OʼReilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg 2014;260:142-8. [Crossref] [PubMed]
- Casadei R, Di Marco M, Ricci C, et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: A single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg 2015;19:1802-12. [Crossref] [PubMed]
- Golcher H, Brunner TB, Witzigmann H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol 2015;191:7-16. [Crossref] [PubMed]
- Okano K, Suto H, Oshima M, et al. A prospective phase II trial of neoadjuvant S-1 with concurrent hypofractionated radiotherapy in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol 2017;24:2777-84. [Crossref] [PubMed]
- Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer 1980;46:1945-9. [Crossref] [PubMed]
- Whittington R, Solin L, Mohiuddin M, et al. Multimodality therapy of localized unresectable pancreatic adenocarcinoma. Cancer 1984;54:1991-8. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7. [Crossref] [PubMed]
- Assifi MM, Lu X, Eibl G, et al. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery 2011;150:466-73. [Crossref] [PubMed]
- Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol 2012;19:1644-62. [Crossref] [PubMed]
- D'Angelo FA, Antolino L, La Rocca M, et al. Adjuvant and neoadjuvant therapies in resectable pancreatic cancer: A systematic review of randomized controlled trials. Med Oncol 2016;33:28. [Crossref] [PubMed]
- Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: Systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- D'Angelo F, Antolino L, Farcomeni A, et al. Neoadjuvant treatment in pancreatic cancer: Evidence-based medicine? A systematic review and meta-analysis. Med Oncol 2017;34:85. [Crossref] [PubMed]
- Dhir M, Malhotra GK, Sohal DPS, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World J Surg Oncol 2017;15:183. [Crossref] [PubMed]
- Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017;6:1201-19. [Crossref] [PubMed]
- Wolff RA. Adjuvant or neoadjuvant therapy in the treatment in pancreatic malignancies where are we? Surg Clin North Am 2018;98:95-111. [Crossref] [PubMed]
- Sutton JM, Abbott DE. Neoadjuvant therapy for pancreas cancer: past lessons and future therapies. World J Gastroenterol 2014;20:15564-79. [Crossref] [PubMed]
- Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:2541-56. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Barnes CA, Krepline AN, Aldakkak M, et al. Is adjuvant therapy necessary for all patients with localized pancreatic cancer who have received neoadjuvant therapy. J Gastrointest Surg 2017;21:1793-803. [Crossref] [PubMed]
- de Geus SWL, Kasumova GG, Eskander MF, et al. Is neoadjuvant therapy sufficient in resected pancreatic cancer patients? A national study. J Gastrointest Surg 2018;22:214-25. [Crossref] [PubMed]
- Stratford JK, Bentrem DJ, Anderson JM, et al. A six-gene signature predicts survival of patients with localized pancreatic ductal adenocarcinoma. PLoS Med 2010;7. [Crossref] [PubMed]
- Nejati R, Goldstein JB, Halperin DM, et al. Prognostic significance of tumor-infiltrating lymphocytes in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemotherapy. Pancreas 2017;46:1180-7. [Crossref] [PubMed]
Cite this article as: Gong J, Chuang J, Hendifar A, Tuli R. Neoadjuvant therapy in upfront resectable pancreatic cancer: current evidence and future considerations. Ann Pancreat Cancer 2018;1:19.

