
Diagnostic intervals and pancreatic ductal adenocarcinoma (PDAC) resectability: a single-center retrospective analysis
Introduction
Pancreatic Ductal Adenocarcinoma (PDAC) is currently the fourth leading cause of cancer-related death in the US and is predicted to rise to second by 2020 (1). For patients diagnosed with PDAC, surgical resection is the only chance for long term survival and potentially curative therapy (2). However, only 15–20% of cases are amenable to surgical resection due to local extension or metastasis (3). The median survival for patients with unresectable disease is less than one year (4).
PDAC is a relatively aggressive tumor with the potential to progress to an advanced stage quickly. Metastases often occur early in the course of disease, most often to the liver, at which point the disease is usually incurable (5). The estimated time for a PDAC tumor to progress from a T1 to a T4 stage is estimated to be approximately 14 months (6). Another factor that contributes to the late presentation of PDAC is the lack of a safe and effective screening strategy. Symptoms of PDAC often do not appear until the later stages of progression and may be triggered by the presence of symptomatic metastasis (7). In addition, symptoms of PDAC are often nonspecific, with an intermittent course that can be falsely reassuring to patients (8). When patients present to medical attention with symptoms that could be consistent with PDAC, many alternative diagnoses must also be considered. The workup for patients is not standardized and multiple follow-up appointments and studies are often required before a final diagnosis is reached (9). These factors are thought to contribute to diagnostic delays and unnecessary healthcare expenditures in the workup of PDAC (10,11).
Prior research has investigated the effects of diagnostic delays on the resectability of breast, colon, and bladder cancer, however, little is known about the impact of such delays on the surgical resectability of PDAC (12). In this retrospective cohort study, we investigated the relationship between the time it takes to present, diagnose and treat the disease and the likelihood of upfront surgical resection for patients with symptomatic PDAC.
Methods
Study cohort
All patients who were evaluated for PDAC at the Center for Pancreas Cancer at Johns Hopkins in 2014 were reviewed using the Johns Hopkins Pancreas Database, a repository of patient clinical data. Patients were included if they presented with symptoms, were diagnosed on the bases of clinical testing arising from these symptoms and had clear documentation of medical presentation and tests. Patients who were asymptomatic and diagnosed from an incidental finding were excluded from analysis. We also excluded any patients for whom either the initial medical presentation for symptoms of PDAC, or workup of tests performed to arrive at the diagnosis and treatment, was incomplete. This study was approved by the Institutional Review Board at Johns Hopkins Hospital.
Data collection
Demographic information on patient age, sex, race, tumor histology, clinic of diagnosis, date of diagnosis and stage of PDAC at diagnosis were collected directly from the database. The date and type of presenting symptoms, initial medical visit, treatment, and diagnostic tests were collected by a single investigator (AB Deshwar) through retrospective review of the patient’s physician encounter notes and imaging documentation. The date and type of the symptoms were extracted from the physician’s note. The date of diagnosis was obtained from data available through the Pancreatic Tumor Registry, defined as the earliest date a primary cancer was diagnosed clinically or microscopically by a recognized medical practitioner. The date of treatment was extracted from patient operative and encounter notes, defined as either the date of surgery or the first day that chemotherapy or radiation treatment began. All tests and procedures that were performed on the patient from their first medical presentation up until, but not including, the first day of treatment for PDAC were recorded.
The course of diagnosis and treatment was divided into three intervals: patient, diagnostic, and treatment intervals. The patient interval was defined as the time from the first onset of PDAC symptoms to the first medical visit to investigate these symptoms. The diagnostic interval was defined as the time from first medical visit to the time of clinical or pathologic diagnosis of PDAC (whichever came first). The treatment interval was defined as the time from diagnosis to first treatment for PDAC.
Presenting symptoms were then grouped into 16 categories based upon shared characteristics: abdominal pain, back pain, bloating, change in stools, chest pain, chills/fever, discolored urine, early satiety, fatigue, gastric reflux, lower extremity edema, nausea, pruritus, vomiting and weight loss.
Tests were categorized into one of 12 groupings based upon modality and relevance towards the workup of PDAC: CT abdomen, CT other, MRI abdomen, MRI other, endoscopic ultrasound (EUS) and/or fine needle aspiration (FNA), esophagogastroduodenoscopy (EGD)/colonoscopy, endoscopic retrograde cholangiopancreatography (ERCP), X-Ray, ultrasound, nuclear medicine, fluoroscopy and other [e.g., biopsy of sites unrelated to PDAC diagnosis and gastrointestinal (GI) function studies]. Diagnostic tests were defined as all imaging and procedures performed from first medical presentation after experiencing symptoms of PDAC, up to and including the day of their diagnosis. Treatment planning tests were defined as all imaging and procedures performed after the date of their diagnosis of PDAC until the day before the patients first treatment.
Statistics
The demographic and disease characteristics of the cohort were summarized using medians with interquartile ranges (IQR, 1st–3rd quartile ranges) for continuous variables and counts with proportions for categorical variables. Shaded bar charts and timelines were used to graphically summarize the timing of the patient, diagnostic, and treatment intervals. Fisher’s exact tests and Wilcoxon rank-sum tests were used to compare categorical and continuous outcomes, respectively, between subgroups of interest. Univariable and multivariable logistic regression was used to assess the impact of demographic and clinical characteristics as well as interval durations on the odds of surgical resection and stage at diagnosis.
Results
Study cohort
Of 453 charts with PDAC that were reviewed, 337 were excluded from the analysis: 283 had an unclear documentation of initial medical presentation or an incomplete progression of tests leading to up to diagnosis and treatment, and 54 had asymptomatic (incidentally diagnosed) disease. The remaining 116 patients met our inclusion criteria for symptomatic PDAC (Table 1). At the time of diagnosis, 7 patients (6%) had stage 1, 53 (46%) had stage 2, 24 (21%) had stage 3, and 32 (28%) had stage 4 disease. The median interval from the beginning of patient symptoms to the first day of treatment for all patients was 74 days (IQR: 45–131 days) (Figure 1). The patient, diagnostic, and treatment intervals for each of the patients included in the analysis is shown in Figure 1. The median number of tests performed from initial medical presentation until treatment was 8 (IQR: 6–11).
Table 1
| Characteristics | Summary statistic |
|---|---|
| Age at 1st medical appointment, median [IQR] (years) | 65 [57–72] |
| Gender, n [%] | |
| Female | 50 [43] |
| Male | 66 [57] |
| Race, n [%] | |
| White | 94 [81] |
| African American | 16 [14] |
| Other | 6 [5] |
| Number of symptoms at presentation | |
| Overall, median [IQR] | 2 [2–4] |
| By type, n [%]* | |
| Abdominal pain | 81 [70] |
| Change in stools | 39 [34] |
| Jaundice | 34 [29] |
| Back pain | 27 [23] |
| Discolored urine | 27 [23] |
| Weight loss | 27 [23] |
| Nausea | 19 [16] |
| Early satiety | 13 [11] |
| Vomiting | 10 [9] |
| Pruritis | 9 [8] |
| Fatigue | 8 [7] |
| GERD | 7 [6] |
| Bloating | 4 [3] |
| Chest pain | 3 [3] |
| Chills | 2 [2] |
| Lower extremity edema | 1 [1] |
| Number of procedures, median [IQR] | |
| Diagnostic | 5 [3–7] |
| Treatment planning | 4 [1–6] |
| Stage at diagnosis, n [%] | |
| 1 | 7 [6] |
| 2 | 53 [46] |
| 3 | 24 [21] |
| 4 | 32 [28] |
| Initial treatment, n [%] | |
| Surgical resection | 38 [33] |
| Chemotherapy | 76 [66] |
| Radiation therapy | 2 [1] |
*, Multiple symptoms are possible for each individual so the total percent will be greater than 100%. IQR, interquartile range; PDAC, pancreatic ductal adenocarcinoma; GERD, gastroesophageal reflux disease.
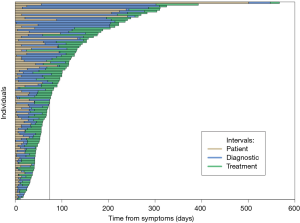
Patient interval
The median patient interval was 14 days (IQR: 6–30 days) and 89 (77%) patients presented to a physician within 1 month after first reporting experiencing symptoms. At initial medical presentation, patients reported a median of 2 (IQR: 2–4) symptoms potentially related to the eventual diagnosis of PDAC. The most common symptoms were abdominal pain (70%), a change in stool habits (34%), and jaundice (29%). Patients with abdominal pain waited significantly longer until their first medical visit as compared to those without (medians: 15.0 vs. 7.0 days, P=0.003). In contrast, patients with jaundice had a shorter wait time compared to those without (medians: 10.0 vs. 14.5 days, P=0.035). No other symptom types were significantly associated with the duration of the patient interval (P>0.05).
Diagnostic interval
The median diagnostic interval was 22 days (IQR: 8–46 days) and 92 (79%) patients were diagnosed with PDAC within 2 months after their first presentation to a physician. All 116 individuals had at least 1 test performed between the first medical visit and diagnosis (median 5, IQR: 3–7, range: 1–12). The most common tests performed were abdominal CT (175, 29%), ultrasound (76, 13%), and EUS (12%). We also examined the last test performed immediately prior to a diagnosis of PDAC being made. The most common test was EUS/FNA (59, 30%) followed by abdominal CT (52, 26%).
Treatment interval
The median treatment interval was 25 days (IQR: 15–35 days). A total of 92 (79%) individuals had additional procedures during the treatment interval, i.e., after their diagnosis but prior to treatment. The median number of treatment planning tests per individual was 4 (IQR: 1–6, range: 0–19). Abdominal CT (n=152, 33%) and Other CT (n=139, 30%) made up the majority of treatment planning tests (Table 2).
Table 2
| Test type | Number of diagnostic tests, n [%] | Last testing prior to diagnosis, n [%] | Number of treatment planning tests, n [%] |
|---|---|---|---|
| Abdominal CT | 175 [29] | 52 [26] | 152 [33] |
| Abdominal MRI | 49 [8] | 4 [2] | 7 [2] |
| EGD/colonoscopy | 32 [5] | 2 [1] | 5 [1] |
| ERCP | 47 [8] | 18 [9] | 25 [5] |
| EUS/FNA | 74 [12] | 59 [30] | 30 [7] |
| Fluoroscopy | 4 [1] | 1 [1] | 18 [4] |
| Nuclear medicine | 9 [1] | 4 [2] | 16 [3] |
| Other | 18 [3] | 11 [6] | 12 [3] |
| Other CT | 61 [10] | 27 [14] | 139 [30] |
| Other MRI | 5 [1] | 0 | 0 |
| US | 76 [13] | 6 [3] | 17 [4] |
| X-ray | 53 [9] | 15 [8] | 37 [8] |
PDAC, pancreatic ductal adenocarcinoma; EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; FNA, fine needle aspiration; CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasound.
Patient, diagnostic, and treatment intervals and surgical resectability
A total of 38 patients (33%) received upfront surgery for treatment of PDAC and 78 (67%) received nonsurgical upfront treatment. Although the decision to proceed to upfront surgery was made on a case by case basis by the consulting surgeon, as a general practice purely resectable pancreas cancers in operative candidates with a CA19-9 <200 were routinely provided upfront surgery. Patients with locally advanced disease, high CA19-9s, or high-risk medical comorbidities were generally provided with nonsurgical treatment. Of those who received upfront surgery, 34 (89%) had stage I or II disease whereas only 26 (33%) of patients who received nonsurgical upfront treatment had stage I or II disease. There was no significant association between age at the first medical visit or gender and the odds of upfront surgical resection (P>0.05, Table 3). However, non-white patients had lower odds of upfront surgical resection than those who were white [adjusted odds ratio (aOR): 0.09, 95% confidence interval (CI): 0.004–0.480, P=0.023]. Patients treated with upfront surgery underwent fewer tests, both diagnostic (median: 4 vs. 5, P=0.61) and treatment planning (median: 2 vs. 4, P<0.001), then those who did not receive upfront surgery.
Table 3
| Risk factor | No upfront surgery (n=78) | Upfront surgery (n=38) | Unadjusted odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI) | Adjusted P value |
|---|---|---|---|---|---|---|
| Age at 1st medical, median [IQR] (years) | 64 [57–70] | 67 [54–76] | 1.01 (0.97–1.05) | 0.480 | 1.00 (0.96–1.05) | 0.850 |
| Male, n [%] | 45 [58] | 21 [55] | 0.91 (0.41–2.00) | 0.800 | 0.91 (0.36–2.24) | 0.830 |
| Non-white, n [%] | 21 [27] | 1 [3] | 0.07 (0.004–0.380) | 0.012 | 0.09 (0.004–0.480) | 0.023 |
| Diagnosis ≤60 days, n [%] | 23 [29] | 37 [97] | 15.47 (3.04–283.00) | 0.009 | 15.68 (2.95–291.00) | 0.009 |
| Medical visit ≤30 days, n [%] | 55 [71] | 34 [89] | 3.55 (1.23–12.91) | 0.030 | 3.41 (1.08–13.20) | 0.050 |
PDAC, pancreatic ductal adenocarcinoma; IQR, interquartile range; CI, confidence interval.
We examined the relationship between patient, diagnostic, and treatment intervals and surgical resectability (Table 3). The odds of resection were more than 3 times higher for those who had a medical visit within 30 days of symptom onset (aOR: 3.41, 95% CI: 1.08–13.20, P=0.050). The median patient interval for those receiving upfront surgery was 10 days (IQR: 6–21 days) as compared to 16 days (IQR: 7–40 days) for those without upfront surgery. Individuals diagnosed within 60 days of the first medical visit had higher odds of upfront surgical resection (aOR: 15.68, 95% CI: 2.95–291.00, P=0.009) (Figure 2). The median diagnostic interval for those receiving upfront surgery was 18 days (IQR: 6–28 days) as compared to 30 days (IQR: 11–72 days) for those who did not receive upfront surgery. The odds of receiving surgery did not differ significantly for those treated within 30 days of diagnosis after adjusting for other risk factors, including the patient and diagnostic intervals (aOR: 1.81, 95% CI: 0.68–5.01, P=0.240).
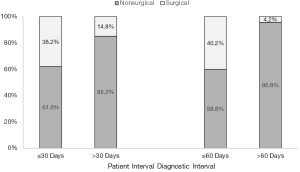
Discussion
We find that patients who wait 30 days or less to present to medical attention after the start of PDAC symptoms, and patients with a diagnostic interval for PDAC after presentation to medical attention of 60 days or less, have increased odds of receiving upfront surgical resection for PDAC. These associations are hypothesis generating, but suggest that delays in PDAC diagnosis may lead to the identification of PDAC at a more advanced stage, when it is less likely to be surgically resectable. Alternatively, advanced PDAC may present with more nonspecific findings than localized PDAC, resulting in longer diagnostic intervals. Our observation that non-white participants may be less likely to receive upfront surgical resection for PDAC could be a reflection of barriers to care, and warrants further investigation. We did not identify any significant association between length of the treatment interval and the odds of upfront surgical resection. This may be because treatment plans are often established at the beginning of the treatment interval and are unlikely to change despite the passage of additional time.
Prior studies across multiple tumor types investigating the relationship between interval lengths in patient care and clinical outcomes have yielded mixed results (12). There have only been three previous studies that have investigated a component of the diagnostic interval and patient outcomes in PDAC. Two of these studies identified a positive association between shorter intervals and improved patient outcomes (13,14), and one found no association (15). Gobbi et al. (13) found a positive association between the “time to diagnosis” (symptom onset–diagnosis) and survival and Raptis et al. (14) found a shorter “pre-hospital” delay (symptom onset–referral to a specialist) to be positively associated with survival. McLean et al. (15) found no association between “wait times” (symptoms–surgical consultation and surgical consultation–procedure) on resectability or survival. However, directly comparing these studies to each other, or to the present investigation has some challenges.
To be able to isolate the impact of the patient, diagnostic and treatment intervals respectively, we followed the protocols for intervals set out by the Aarhus statement on improving designs of early cancer diagnosis studies (16). Historically, these intervals have not been consistently defined, making comparison difficult. For example, these three previous studies used the date symptoms began to begin the diagnostic interval; the result being a measurement that combines what we identified as the patient (date of first symptoms–first medical appointment) and diagnostic (first medical appointment–diagnosis of PDAC) intervals. Instead, using the date of first medical presentation allows for a more consistent and representative anchor from which to begin the diagnostic interval. A well-defined patient vs. diagnostic interval is crucial, to be able to contextualize findings such as Lyratzopoulos et al. observing that 41% of patients eventually diagnosed with pancreatic cancer, visited their general practitioner three or more times before hospital referral; second only to multiple myeloma amongst major cancers (9). This parallels findings in previous investigations, observing that PDAC patients are often subject to a large variance in the diagnostic workup of testing that they receive, and this often has correlates to greater delays and higher healthcare costs (10,11).
A strength of this investigation includes the use of a large and well-characterized cohort of PDAC patients. However, this study also has certain limitations. We cannot exclude the possibility of selection or recall bias in our study, resulting from the retrospective nature of the data, the study selection criteria, and the reliance on patient self-report to their physicians for ascertainment of the patient interval. In addition, causality cannot be inferred from the observed relationship between patient and diagnostic intervals and the odds of upfront surgical resection. Finally, the majority of the patients in this cohort were treated at a single tertiary referral center, which may limit the generalizability of our findings. Since it is not ethically feasible to delay surgery for patients with PDAC to determine the effect of treatment delays on outcomes, additional retrospective analyses will be necessary to confirm our findings. In summary, a patient interval of less than 30 days and a diagnostic interval of less than 60 days for symptomatic PDAC, are associated with a clinically meaningful improved probability of upfront surgical treatment. These data suggest that efforts to reduce delays may lead to improved outcomes in PDAC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.02.01). LZ serves as the unpaid Editor-in-Chief of Annals of Pancreatic Cancer. Jin He serves as an unpaid Section Editor of Annals of Pancreatic Cancer. CW serves as an unpaid editorial board member of Annals of Pancreatic Cancer. Daniel Laheru serves as an unpaid editorial board member of Annals of Pancreatic Cancer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) at Johns Hopkins Hospital (NA_00068179). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kenner BJ, Chari ST, Cleeter DF, et al. Early detection of sporadic pancreatic cancer: strategic map for innovation--a white paper. Pancreas 2015;44:686-92. [Crossref] [PubMed]
- Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol 2015;21:3157-65. [Crossref] [PubMed]
- Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc 1999;50:786-91. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Yu J, Blackford AL, dal Molin M, et al. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 2015;64:1783-9. [Crossref] [PubMed]
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Evans J, Chapple A, Salisbury H, et al. BMJ Open 2014;4:e004215 [Crossref] [PubMed]
- Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012;13:353-65. [Crossref] [PubMed]
- Driedger MR, Dixon E, Mohamed R, et al. The diagnostic pathway for solid pancreatic neoplasms: are we applying too many tests? J Surg Res 2015;199:39-43. [Crossref] [PubMed]
- Cooper M, Newman NA, Ibrahim AM, et al. Unnecessary Tests and Procedures in Patients Presenting with Solid Tumors of the Pancreas. J Gastrointest Surg 2013;17:1218-23. [Crossref] [PubMed]
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112:S92-107. [Crossref] [PubMed]
- Gobbi PG, Bergonzi M, Comelli M, et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 2013;37:186-90. [Crossref] [PubMed]
- Raptis DA, Fessas C, Belasyse-Smith P, et al. Clinical presentation and waiting time targets do not affect prognosis in patients with pancreatic cancer. Surgeon 2010;8:239-46. [Crossref] [PubMed]
- McLean SR, Karsanji D, Wilson J, et al. The effect of wait times on oncological outcomes from periampullary adenocarcinomas. J Surg Oncol 2013;107:853-8. [Crossref] [PubMed]
- Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262-7. [Crossref] [PubMed]
Cite this article as: Deshwar AB, Sugar E, Torto D, De Jesus-Acosta A, Weiss MJ, Wolfgang CL, Le D, He J, Burkhart R, Zheng L, Laheru D, Yarchoan M. Diagnostic intervals and pancreatic ductal adenocarcinoma (PDAC) resectability: a single-center retrospective analysis. Ann Pancreat Cancer 2018;1:13.

