Case analysis of pancreatic cancer treated with FOLFIRINOX in China
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is aggressive, has a poor prognosis, and ranks as the fourth leading cause of cancer-related deaths in the United States (1). In China, 90,100 people were diagnosed with PDAC, and its mortality was 79,400 in 2015 (2). Surgical resection is regarded as the only potentially curative treatment, although <20% of patients with PDAC are eligible for resection upon diagnosis (3). Even after curative resection, most patients will eventually experience recurrence, defined as recurrent pancreatic cancer (RPC). The resection rate of PDAC is limited, and therefore most patients with PDAC have locally advanced pancreatic cancer (LAPC) or metastatic pancreatic cancer (MPC). Liver-metastatic pancreatic adenocarcinoma (LMPC) is the most common subset of MPC. Moreover, patients with PDAC who are eligible for resection are considered to have borderline resectable pancreatic cancer (BRPC) (4). Chemotherapy is one of the main treatments for patients with RPC or LMPC, and neoadjuvant chemotherapy is now advocated for patients with BRPC.
The FOLFIRINOX regimen (comprising 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) represents a remarkable advance in the treatment of PDAC. FOLFIRINOX achieves a statistically significant and clinically conspicuous benefit compared with that of gemcitabine in patients with MPC (5). A comparison of the clinical efficacy of surgery for patients with LAPC or BRPC who did or did not receive neoadjuvant FOLFIRINOX treatment revealed that FOLFIRINOX achieves a significant decrease in tumor size, shorter surgical times, decreased number of positive lymph nodes and perineural invasion as well as a significant increase in overall survival (6). Moreover, FOLFIRINOX is effective for RPC patients, with a response rate to FOLFIRINOX chemotherapy of 11.1%, and a disease control rate of 77.8% (7).
In China, as the hesitance of oncologists to use FOLFIRINOX due to the concern on the toxicity of the regimen and the presumption that Chinese patients could not tolerate FOLFIRINOX, the method of FOLFIRINOX hasn’t been widely used. Here we report our encouraging experience using FOLFIRINOX to treat consecutive Chinese patients with PDAC, including those with BRPC, LMPC, and RPC.
Methods
Patients
Patients histologically diagnosed with PDAC who received FOLFIRINOX from July 2016 to July 2018 were eligible for inclusion. Three patient was diagnosed with BRPC, three with LMPC, and four with RPC. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, with normal bone marrow and renal function. Liver function had to improve (bilirubin <1.5-times the upper limit of the normal range) before chemotherapy was administered. Patients’ characteristics are presented in Table 1. Patients granted their written informed consent to undergo FOLFIRINOX treatment during this study, which was approved by the Ethics Committee of our hospital.
Table 1
| No. | Sex | Age | Diag | ECOG score | Aim of treatment | FOLFIRINOX cycles | FOLFIRINOX dose | Clinical efficiency | Toxicity | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 45 | BRPC | 0 | Neoadjuvant therapy | 9 (4 before OP and 5 after OP) | 100% | PR, operation | Vomiting and nausea, grade 2 | Alive, 11 months |
| 2 | F | 29 | BRPC | 1 | Neoadjuvant therapy | 5 (4 before OP and 1 after OP) | 80% | PR, operation | Vomiting and nausea, grade 2 | Alive, 5 months |
| 3 | M | 69 | BRPC | 0 | Neoadjuvant therapy | 3 | 100% | PR, operation | Vomiting and nausea, grade 1 | Alive, 3months |
| 4 | M | 59 | LMPC | 0 | Converse | 11 (5 before OP and 6 after OP) | 100% to 80% | PR, operation | Febrile neutropenia, grade 3 | Alive, 12 months |
| 5 | M | 51 | LMPC | 0 | Converse | 12 | 100% | PR, FX treatment | Neutropenia, grade 2 | Died, 11 months |
| 6 | F | 60 | LMPC | 1 | Converse | 12 | 80% | PR, FX treatment | Neutropenia, grade 2 | Alive, 8 months |
| 7 | M | 46 | RPC | 0 | Second-line therapy | 7 | 100% | SD,CA19-9 decrease >60% | None | Alive, 12 months |
| 8 | M | 65 | RPC | 0 | Second-line therapy | 9 | 100% | PD, CA19-9 decrease >60% | Neutropenia, grade 2 | Alive, 14 months |
| 9 | M | 62 | RPC | 0 | Second-line therapy | 6 | 100% | PD, CA19-9 decrease >60% | Neutropenia, grade 2 | Died, 18 months |
| 10 | M | 76 | RPC | 1 | Second-line therapy | 1 | 80% | PD, CA19-9 stable | Neutropenia, grade 2 | Died, 12 months |
FOLFIRINOX therapy
The standard treatment using FOLFIRINOX (85 mg/m2 oxaliplatin, 180 mg/m2 irinotecan, 400 mg/m2 leucovorin, and 400 mg/m2 5-fluorouracil given as a bolus followed by 46 h continuous infusion of 2,400 mg/m2 5-fluorouracil) was administered every 2 weeks to seven patients, as originally described (5). Three patients received an initial 80% dose, according to their ECOG performance status. During FOLFIRINOX treatment, dose modifications were made at the treating physician’s discretion based on patient’s ECOG performance status or on observed toxicity.
Results
Clinical efficacy
Ten patients underwent FOLFIRINOX therapy, seven received an initial full dose of FOLFIRINOX, and three received an 80% dose, according to their ECOG performance status. One patient with RPC refused to accept further chemotherapy after one cycle. The other nine patients received at least three cycles. A significant decrease in tumor size was detected in three patients with BRPC after neoadjuvant therapy, and they subsequently underwent radical resection. Shrinkage of the tumor and reduction of liver metastases were observed in three patients with LMPC, and one of these patients successfully underwent surgical resection. Two patients with LMPC achieved partial remission (PR) but refused surgery for personal reasons. Three patients who experienced tumor recurrence after gemcitabine-plus-capecitabine treatment received 4–6 cycles of FOLFIRINOX as second-line therapy, which controlled tumor progression. The serum levels of carbohydrate antigen 19-9 (CA19-9) significantly decreased (>60%) in three patients with RPC. The response rate (six patients achieved PR and one achieved stable disease) in nine patients who accepted more than three cycles of FOLFIRINOX therapy was 77.8%.
Adverse events
Neutropenia was the most common adverse event. One patient with LMPC had febrile neutropenia (grade >3) after the second cycle of FOLFIRINOX, after which the dose was reduced to 80%. The other nine patients did not experience serious adverse events (grade >3).
Follow-up
All patients were followed. Three patients with BRPC and one with LMPC, all of whom underwent surgery, received FOLFIRINOX therapy without experiencing tumor recurrence. One patient with LMPC died of brain metastasis and one was undergoing treatment when our manuscript was at the time of writing. One patient with RPC received six cycles of FOLFIRINOX after gemcitabine-plus-capecitabine treatment but abandoned further chemotherapy and died because of tumor progression 5 months later. One patient with RPC, who refused to accept further chemotherapy after one cycle of FOLFIRINOX, was died of extensive metastasis of the tumor. One patient with RPC was receiving treatment without tumor recurrence at this time. One patient with RPC abandoned further treatment after nine cycles of FOLFIRINOX therapy and got multiple liver metastases. Patients’ characteristics, clinical efficiencies, adverse events, and follow-up findings are presented in Table 1.
Case presentation
BRPC
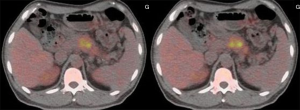
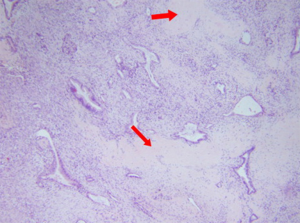
A 45-year-old man presented with a 2-month history of back and abdominal pain, a 4-year history of diabetes mellitus, and a 20-year history of chronic hepatitis B. His serum concentrations of CA19-9 and carcinoembryonic antigen (CEA) were >1,000 and 17.22 U/L, respectively. Other blood values were normal. Enhanced computed tomography (CT) showed that the tumor was located at the neck and body of the pancreas with involvement of the hepatic artery, splenic artery, and superior mesenteric vein (Figure 1). Positron-emission tomography-CT (PET-CT) showed a mass with high metabolic activity in the same region but did not detect a metastatic lesion (Figure 2). Endoscopic ultrasonography-guided biopsy confirmed the diagnosis of pancreatic adenocarcinoma. Four cycles of FOLFIRINOX were administered. During treatment, the patient experienced only grade-2 adverse events (vomiting and nausea). After chemotherapy, we evaluated the enhanced CT data and found that the size of the tumor was obviously smaller (Figure 1). A distal pancreatectomy and splenectomy was performed and histopathology revealed a well-differentiated PDAC with no detectable metastasis in the regional lymph node (0/15), and significant necrosis and fibrosis were present in the tumor (Figure 3). After operation the patient accepted five cycles of FOLFIRINOX treatment and was alive 8 months after surgery with no detectable recurrence.
LMPC
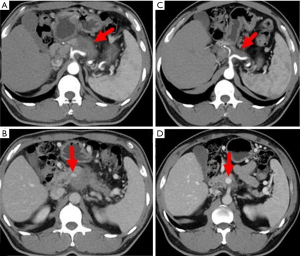
A 59-year-old man presented with a 1-month history of abdominal distension as well as jaundice of the skin and sclera for 18 days. He had a 10-year history of diabetes mellitus and hypertension. He had abnormally high concentrations of biomarkers of liver tumors and function (CA19-9, 264.1 U/L; CEA, 8.0 U/L; alanine aminotransferase, 144 IU/L total bilirubin, 415 µmol/L; and direct bilirubin, 223 µmol/L). Magnetic resonance cholangiopancreatography (MRCP) revealed bile duct dilatation and a mass at the head of the pancreas. Enhanced CT detected a metastatic lesion in the right lobe of the liver (Figure 4). Endoscopic ultrasonography-guided biopsy confirmed the diagnosis of pancreatic adenocarcinoma. Percutaneous transhepatic cholangio drainage was then performed to reduce jaundice, which was followed by five cycles of FOLFIRINOX. During the second cycle of chemotherapy, a grade-3 adverse event (febrile neutropenia) occurred. Therefore, the remaining three treatment cycles used an 80% dose of FOLFIRINOX. After chemotherapy concluded, enhanced CT revealed obvious reduction of the pancreatic and liver lesions (Figure 4). The patient subsequently underwent pancreaticoduodenectomy, during which intraoperative brightness mode ultrasound did not detect suspicious liver lesions. Histopathology, which revealed a PDAC that exhibited intermediate differentiation, detected two of 31 regional lymph nodes with metastasis. After operation the patient accepted six cycles of FOLFIRINOX treatment and was alive 12 months after diagnosis without detectable recurrence.
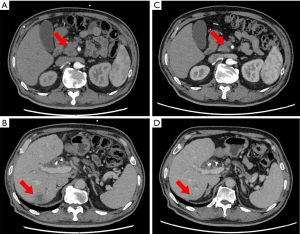
RPC
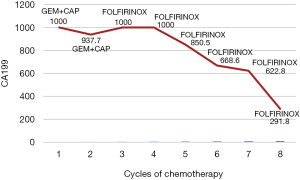
A 65-year-old man presented with a 5-day history of abdominal distension. MRCP and enhanced CT showed a mass located at the head of the pancreas. Endoscopic ultrasonography-guided biopsy confirmed the diagnosis of pancreatic adenocarcinoma, and Pancreaticoduodenectomy was subsequently performed. Histopathology showed a PDAC with intermediate differentiation but did not detect regional lymph node metastasis (0/19). After surgery, the patient received two cycles of gemcitabine combined with capecitabine. However, his high CA19-9 concentration persisted, and PET-CT detected tumor recurrence at the region of bilioenteric anastomosis and the fifth segment of the liver. PET-CT showed a mass with high metabolic activity at the anastomotic biliary-intestinal region and segment 5 of the liver. Therefore, we switched to FOLFIRINOX treatment. The patient’s CA19-9 concentration decreased more than 2-fold after six cycle of FOLFIRINOX treatment (Figure 5). This patient abandoned further chemotherapy after nine cycle of FOLFIRINOX treatment and got multiple liver metastasis. He was alive 14 months since diagnosed.
Discussion
Here we present our experience with FOLFIRINOX chemotherapy administered to patients with pancreatic cancer at our institution. Pancreatic cancer subtypes included BRPC, LMPC, and RPC. Specifically, we report the clinical efficacy of FOLFIRINOX for Chinese patients with different clinical stages of PDAC. Although the number of patients was limited, the efficacy of FOLFIRINOX was inspiring.
For decades, fluorouracil and subsequently gemcitabine regimens were the preferred palliative first-line options for patients with advanced pancreatic cancer. In 2010, FOLFIRINOX was shown as an effective treatment for patients with MPC compared with gemcitabine (5). FOLFIRINOX is now considered the first-line chemotherapy regimen for MPC. However, FOLFIRINOX is associated with a high incidence of adverse events such as neutropenia, fatigue, anemia, peripheral sensory neuropathy, and diarrhea. In China, FOLFIRINOX hasn’t been widely used as the hesitance of oncologists for the toxicity of the regimen and the presumption that Chinese patients could not tolerate FOLFIRINOX. In our case series, the most common adverse event was neutropenia (grade 2), and one patient experienced febrile neutropenia, a grade >3 adverse event. We conclude therefore that to reduce toxicity, the dose of FOLFIRINOX should be modified according to the patient’s ECOG performance status.
Numerous studies attempted to balance the efficacy and toxicity of FOLFIRINOX (8-11). For example, one study determined the optimal relative dose intensity (RDI, %) of FOLFIRINOX, with the aim of preserving tumor responses and reducing toxicity (12). The investigators developed a modified Hryniuk model and the defined cumulative RDI of 133 patients with advanced PDAC. They found that the cRDI values for FOLFIRINOX should be >70% and >55%, respectively, to preserve optimal response and disease control rates (12). Here we found that most patients with PDAC tolerated the toxicity of FOLFIRINOX if it was dose-adjusted (80% to 100% of initial dose). Further studies of a larger number of patients are required to confirm the appropriate dose of FOLFIRINOX.
Our present patients with BRPC or LMPC underwent successful surgery, though two refused for personal reasons. Consistent with our present study, a meta-analysis found that R0 resection is possible for 63.5% of patients with BRPC who receive FOLFIRINOX therapy (13). Further, two patients with LMPC treated with FOLFIRINOX achieved complete remission and subsequent R0 resection of primary tumors (14). For patients with RPC, we found that there was a significant decrease (>60%) in CA19-9 concentrations after 4–6 cycles of FOLFIRINOX chemotherapy. These findings are consistent with those of a study that observed ≥20% decreases in CA19-9 concentrations within 8 weeks, accompanied by improved median OS, PFS, and objective responses (15).
Therefore, our present findings are quite inspiring. Further, patients with RPC initially received gemcitabine-plus-capecitabine, which we changed to FOLFIRINOX when relapse or metastasis was detected. Therefore, this strategy may benefit patients who are resistant to gemcitabine-based therapy. Though there is no recommended second-line treatment for pancreatic cancer, a phase I study indicates that second-line FOLFIRINOX is an effective treatment when gemcitabine-based chemotherapy fails (16).
In summary, we found that FOLFIRINOX chemotherapy was safe and effective for Chinese patients with advanced pancreatic cancer who have good ECOG performance status. To reduce toxicity, the dose of FOLFIRINOX should be adjusted according to a patient’s performance status.
Acknowledgments
We thank Liwen Bianji, Edanz Group China (
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The report was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This report was approved by the Institutional Review Board of China-Japan Friendship Hospital (No. 2015-44) and informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267 [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Sakamoto T, Takeda Y, Ohmura Y, et al. The Treatment Outcomes of FOLFIRINOX for Unresectable and Recurrent Pancreatic Cancer. Gan To Kagaku Ryoho 2017;44:1751-53. [PubMed]
- Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016;114:737-43. [Crossref] [PubMed]
- Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311-15. [Crossref] [PubMed]
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153-59. [Crossref] [PubMed]
- Ghorani E, Wong HH, Hewitt C, et al. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology 2015;89:281-87. [Crossref] [PubMed]
- Lee JC, Kim JW, Ahn S, et al. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: Using cumulative relative dose intensity. Eur J Cancer 2017;76:25-33. [Crossref] [PubMed]
- Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 2015;44:515-21. [Crossref] [PubMed]
- Schneitler S, Kröpil P, Riemer J, et al. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J Gastroenterol 2015;28:6384-90. [Crossref] [PubMed]
- Robert M, Jarlier M, Gourgou S, et al. Retrospective Analysis of CA19-9 Decrease in Patients with Metastatic Pancreatic Carcinoma Treated with FOLFIRINOX or Gemcitabine in a Randomized Phase III Study (ACCORD11/PRODIGE4). Oncology 2017;93:367-76. [Crossref] [PubMed]
- Kobayashi N, Shimamura T, Tokuhisa M, et al. Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure. Medicine 2017;96:e6769 [Crossref] [PubMed]
Cite this article as: Si S, Yang Z, Tan H, Sun Y, Liu X, Xu L, Liu L, Zhou W, Huang J. Case analysis of pancreatic cancer treated with FOLFIRINOX in China. Ann Pancreat Cancer 2018;1:31.