How to teach and train laparoscopic pancreatoduodenectomy
Introduction
Background on the procedure
Pancreatoduodenectomy (PD) is the treatment of choice for cancers and premalignant cysts in the pancreatic head and periampullary region. The laparoscopic approach to PD (LPD) was first described in 1994 (1), but gained popularity slowly, possibly due to the extensive laparoscopic dissection, and the technical difficulty of the pancreatic and biliary anastomoses. Additionally, a worldwide survey showed that the most reported reason for not implementing LPD was lack of specific training (2). Developments in surgical expertise, and instrumentation have improved the feasibility of LPD. Nevertheless, only few centers have acquired adequate experience with this complex procedure (2).
The era of the surgical dogma “see one, do one, teach one”, is long gone (3). As reflected by recent reports, the learning curve of LPD is demanding. Dokmak et al. stated an initial learning phase can be achieved after 40 cases (4). The main factors contributing to the learning curve of the LPD are previous experience and annual case volume of the operating surgeon. Speicher et al., using a laparoscopic resection followed by open anastomoses, demonstrated a reduction in operative times after the first 10 LPDs (5). After approximately 50 cases, estimated blood loss levels were lower than those for open PD (OPD) (5). Results, however, vary between centers (4).
In addition to the learning phase, a minimum annual volume could be required. Whereas for OPD a minimum annual volume of 40 cases has shown to improve outcome, a minimal annual volume of 30 LPDs is probably required to obtain similar results as from OPD (6). This is further reflected by the LAELAPS-2 and the LEOPARD-2 trial, where results of LPD centers seem to have deteriorated after randomization halved the case-load frequency (7).
Thus, according to large database analyses, surgical experience of more than 60–80 LPD cases and an annual hospital volume of 22–30 LPD procedures are required to perform LPD with similar outcomes to the open approach (8). Now, which interventions can shorten this learning curve and further improve outcomes?
This review does not aim to set standards for which centers and which surgeons should or should not perform LPD, but rather presents methods of teaching and implementing LPD as safely as possible.
Aim
Our aim is to describe methods of practicing and teaching LPD.
Methods
We describe the steps of LPD and beneficial methods of teaching and practicing each step. The operative steps were described previously (9). Methods of teaching and practicing were found by a literature search as described in the Supplementary. Written informed consent was obtained from the patient for publication of this article and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Methods of teaching and practicing LPD
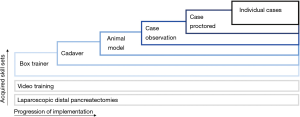
In a survey directed at Hepato-Pancreatico-Biliary (HPB) fellowship directors, Subhas et al. concluded that the case-load for HPB fellows is not sufficient in today’s practice (10). The aim of these fellowships is generally to maximize the experience, e.g., case-load, for fellows, without subjecting patients to their learning curve. Still, laparoscopic box training alone cannot efficiently substitute case load experience. However, acquired laparoscopic skills enable a more specific focus on the complexities of the LPD procedure. The introduction of LPD should be a stepwise approach (3). With essential skills learned at each step and retained by the consecutive step. Preferably, trainees should start with box training of the reconstruction phase, then the resections phase could be performed in cadavers, followed by animate scenario based vascular control and repair, and finally, proctor cases. Meanwhile, video training and performing laparoscopic distal pancreatectomies further benefit the learning retention and preparation for individual cases (see Figure 1).
Resection phase
For the stepwise implementation, the trajectory of practicing and teaching the resection phase requires planning, collaboration, and training resources. Practice with cadaver models and animal models provide a representable platform for preparation. It is important to maintain an individual or joined video library of “reference standard” procedures, as many open source videos lack the adequate surgical performance or use a different approach. Additionally, a sharing platform for videos is ideal to gain advice and feedback from colleagues. The proctor can provide feedback and coaching on video material of the recorded cases or by subsequent visits on-site using a Birkmeyer scoring platform for laparoscopic bariatrics, modified by the University of Pittsburgh Medical Center (UPMC) (11).
Trocar placement and extraction site
Trocar injuries are a noteworthy complication in laparoscopic procedures in general (12). Consequences are severe when not recognized or when inappropriately addressed. In LPD they could potentially cause enteric and vascular injury, yet reported incidence is low (12). One should be familiar with both a Veress needle technique with subsequent 12 mmHg pneumoperitoneum, and, the alternative “open introduction” at the umbilicus (13). As cadaver models lack blood flow, porcine models serve as a training method to practice trocar placement according to both techniques.
Mobilization
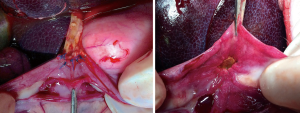
The mobilization is characterized by accessing the lesser sack, exposing the pancreatic head and duodenum by dissecting all avascular planes, and dividing the intestine. Therefore, cadaver training should suffice in practicing this step. For surgeons experienced in colorectal and/or pancreatic tail resection, practicing these steps is warranted only for the Kocherization. Here, the second surgeon should be taught to pull the duodenum to the left side of the patient, and push the colon to the right caudal side of the patient to expose the dissection line (see Figure 2). Exposing and clipping the vessels encountered during this phase is similar to that of smaller, less challenging procedures. Make sure to have seen the approach of this step for aberrant vasculature, e.g., aberrant right hepatic artery from the superior mesenteric artery.
Vascularization & involvement
The vascularization & involvement step is characterized by the dissection of the hepatic ligament, the inferior border of the pancreas and portal dissection. Therefore, training should focus on lymph node harvest, bleeding control, and transection of the gastroduodenal artery. The use of animal models for surgical training is quite common (14). The porcine model has been validated for multiple laparoscopic procedures (15). Perfusion of the vascular structures makes for an ideal setting to become familiar with bleeding control and vascular repair. The porcine model is valuable in practicing this step to reach proficiency, yet falls short in practicing exposing the superior mesenteric vein. The anatomy of the porcine pancreas is such, that its pancreas covers the superior mesenteric vein for only small portion (16). The tunneling of the pancreas should be an essential part of the viewed videos, and should be taught on cadavers, performed during distal pancreatectomy, and performed with guidance of a proctor.
Uncinate process
At this point of the procedure, the specimen ought to find its last attachments to the superior mesenteric vein and superior mesenteric artery. In teaching this section, extensive proctoring is vital. The exposure and identification of vascular structures should be the main focus. Even though options for teaching vascular reconstruction are abundant, vascular reconstruction should be utilized only under exceptional conditions.
The uncinate process step can be divided in two parts, and should be taught accordingly. The first part is venous, the second is arterial. As always, bleeding should be prevented, yet during this phase the threshold for conversion is much lower. This, due to the difficult exposure and limited freedom of movement around the vital vascular structures. During the venous part, the proctor should demonstrate how to control and transect the venous branches from the pancreatic specimen to the superior mesenteric vein; bleeding and avulsion are common here. During the arterial part, the proctor should demonstrate how to distinct the small arteries from lymphatics without entering the pancreatic tissue. Watching videos on this section is very valuable for understanding pitfalls that arise by pulling exposure forces.
Only after 75–100 cases, some authors have demonstrated the feasibility of vascular resection and have advised to reserve it for when the technical limitations of the team have been overcome (4). Unfortunately, proctoring such cases would be unlikely due to the low prevalence of resectable venous involvement. Teaching should aim to discourage laparoscopic vascular resection and reconstruction. Since this opinion is not shared by all, we advise to follow each implementation step. This should look as follows:
- First, videos should demonstrate the preferred technique for clamping and resecting the venous involvement;
- Then, these techniques can be implemented in cadaver training and on the porcine model. Practice wedge resections, patch reconstructions, and end-to-end reconstructions;
- Meanwhile, when encountering potential wedge resections, make sure to clamp and control the superior mesenteric vein and splenic vein. Remember, the inferior mesenteric vein may enter the superior mesenteric vein caudally from the confluence of the superior mesenteric vein and the splenic vein;
- Finally, when encountering the first wedge resection, double check all needs for conversion before deciding to perform the resection. Do not subject the first vascular resection cases to end-to-end reconstruction attempts;
- Afterwards, review the procedure in your library and the proctor should provide feedback.
Specimen extraction and reconstruction phase
Some centers have argued that reconstruction via a mini-laparotomy can be used during the initial learning curve phase (17). The benefits of such an approach remain to be proven, and the incidence of incisional hernias appear similar for the open approach, whereas a Pfannenstiel incision carries a negligible rate of incisional hernias (18). Here, the teaching of the Pfannenstiel incision can be limited to techniques avoiding puncturing of the bowel, e.g., cancel out anti-Trendelenburg while opening the extraction site.
We do not advocate any specific reconstruction technique, since many different centers have demonstrated excellent reports on varying techniques (19). However, reports comparing different techniques could be confounded by surgeon’s preference, and are lacking or indecisive when concerning the laparoscopic approach (20). The authors suggest it is best to adopt the technique acquired from the proctor. Reasonably, every adjustment in the technique can undesirably impact the learning curve.
Pancreatic anastomosis
A novelty for the step-wise implementation is training with artificial organs (see Figure 3). These synthetic tissues were warranted, as the human pancreas has a unique structure and anatomy. In our experience, the porcine model is without a main pancreatic duct, most likely due to a young age at a representative body mass. Furthermore, the fresh frozen cadaver model is subjected to decay, and auto-phagation of the pancreas. Hence, pancreatic structure stays representative for anastomosis practice only in a small time frame, i.e., the unpreserved post-mortem time: 15–20 hours (see Table 1). The main goal should be to have the main surgeon perform this anastomosis over 5 times before implementing it in the patient setting. In our experience, any procedural gaps of over 2 weeks should be supplemented by anastomosis practice.
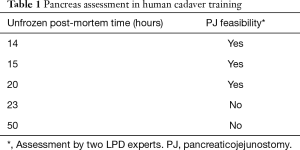
Full table
Hepaticojejunostomy
Similar to the pancreaticojejunostomy, artificial organs comprise the first step in teaching laparoscopic hepaticojejunostomy and could be substituted by cadaver model training. Additional transfer of skill to the animate setting should be attempted in the porcine model, since this provides a means of assessing the anastomosis for leaks and imperfections (see Figure 4). The main focus of teaching should be to standardize anastomosis methods according to duct characteristics, prepare oneself for small side branch anastomoses, and to handle precision in spite of the movement through breathing and the projected pulsation of the hepatic artery.
Gastro-/duodenojejunostomy
Although gastric- and duodenal anastomoses are common and seemingly simple, they know many major pitfalls. They are not easily taught on the porcine model due to a relatively thick stomach wall with a relatively thin jejunal wall. In the cadaver model, the enterotomies allow free flow of intestinal contents. The teaching should focus on performing inverting stitches and, when stapling, gentle compression of the tissues, and correct measurement of the minimal length for the roux-limb. This can be done with artificial organ models, video review, and proctoring.
Conversion
Teachings on conversion during LPD should focus on conveying the right paradigm. There should be a low threshold for conversion during LPD and conversion should not be seen as a complication. Unsurprisingly, conversion is reported ranging from 2% to 14% (21). One should constantly question whether the patient would benefit from conversion to an open approach. Conversion should definitively not be felt as surgical failure. Urgent conversion can be practiced on the porcine model: the assisting surgeon compresses or holds the bleeding defect with a laparoscopic instrument, while laparotomy is performed.
Discussion
In 2014, the Dutch Pancreatic Cancer Group (DPCG) introduced the Longitudinal Assessment and Safe Implementation of Laparoscopic Pancreatic Surgery (LAELAPS) program in The Netherlands (22). LEALAPS-1 and -2, were designed to train pancreatic surgeons in laparoscopic distal pancreatectomy (LDP) and LPD. Surgeons received a detailed description of the procedures, video training, and on- and off-site proctoring by an experienced laparoscopic pancreatic surgeon. LEALAPS-1 was followed by a randomized controlled trial comparing LDP and open distal pancreatectomy (ODP): LEOPARD-1 (23). Which showed faster functional recovery, shorter hospital stay, less intraoperative blood loss, and less delayed gastric emptying for LDP compared to ODP (23). LAELAPS-2 was followed by the recently completed LEOPARD-2 randomized controlled trial comparing LPD and OPD (24). This trial was stopped early because of safety concerns without demonstrable benefits for patients (see Table S1 in the Supplementary for an overview of trial results). LEALAPS-3, is a nationwide implementation program for robot-assisted PD using virtual reality simulation exercises, video training, advanced suturing and anastomosis training, and on- and off-site proctoring. This program is being performed in close collaboration with the surgeons of the UPMC robotic pancreas program (25).
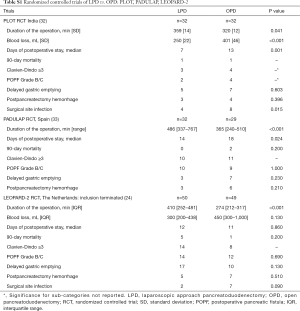
Full table
In a Canadian survey, more than 75% (n>45) of respondents felt that PD should only be performed at high volume centers (26). Hospital volume is indeed significantly associated with improved LPD outcomes. As a minimum threshold of 22–30 LPD procedures per year should be maintained to have enough and continuous exposure and experience (27). It should be stated however, that on-site training facilities could have biased the data backing up this minimum threshold, e.g., at our hospital, we organize the annual advanced minimally invasive pancreatic surgery course for the European-Consortium on Minimally Invasive Pancreatic Surgery (www.E-MIPS.org). Such courses provide further development of knowledge and experience of the surgeons as a group, and address training to maintain the plateau phase of the learning curve. Another favorable factor of bias could be the previous experience in open surgery and LDP. Therefore, the threshold for the minimal annual cases is still under debate, yet there should definitively be a minimum. And, even with vast amounts of previous experience, simulation training for the reconstructive phase during LPD is essential in completing and retaining the learning curve (28).
While observing operative videos can be an important learning tool, surgical educators should be aware of the low quality of popular videos on open source video platforms such as YouTube and should respect patient privacy policies (29). The expert surgeon, the proctor, should address all required skills and ensure safety monitoring of the potential blind spots of the “student”. Therefore, the proctor should have fully completed his/her own learning curve.
In this review, we demonstrated an evidence-based approach to safe implementation and teaching op LPD. Achurra et al. aimed to reduce the learning curve with ex-vivo acquired skills that were transferred to the operating room (30). The proctor can provide feedback and coaching on video material of the recorded cases or by subsequent visits on-site using a Birkmeyer scoring platform for laparoscopic bariatrics, modified by UPMC (3,11,31). Through our systematic approach to literature search, we were able to provide a detailed overview of current practice- and teaching methods.
Conclusions
Implementing LPD is challenging due to the learning curve, the annual volume required and the complex nature of the procedure. Different reports have shown that outcomes of LPD become similar to OPD, only after surpassing the learning curve and with an annual volume of at least 22–30 LPDs. Although surgical performance naturally differs between practices, centers should focus on minimizing patient’s subjection to their learning curve while implementing LPD. This requires registration and recording of surgical performance, and an extensive, coordinated training program.
A structured training program aims to teach essential retainable skills in a stepwise approach outside the OR setting. Meanwhile, different steps of the procedure or more simple procedures can support experience, e.g., starting with a hybrid approach. Nonetheless, it is wise to start all first procedures under direct guidance of an experienced LPD proctor, and to request feedback and coaching during the learning curve. Thereby working towards a short, safe learning curve.
Supplementary
Methods of literature review
Search strategy
The search strategy focused on three main subjects; education, learning curve, and complications during and after LPD. We searched for articles in CENTRAL, PubMed and EMBASE published from January, 1993, i.e., just before the first report on LPD, until February 2018.
CENTRAL search strategy
#1 MeSH descriptor: [Laparoscopy] explode all trees;
#2 (laparoscop*);
#3 (Pancreatoduodenec*);
#4 #1 or #2;
#5 #3 and #4;
#6 MeSH descriptor: [Intraoperative Complications] explode all trees;
#7 MeSH descriptor: [Postoperative Complications] explode all trees;
#8 #6 or #7;
#9 MeSH descriptor: [Learning Curve];
#10 MeSH descriptor: [Education] explode all trees;
#11 #8 or #9 or #10;
#12 #5 and #11.
Ovid search strategy for PubMed and EMBASE
#1 MeSH descriptor: [Laparoscopy] explode all trees;
#2 (laparoscop*);
#3 MeSH descriptor: [Hand-Assisted Laparoscopy] explode all trees;
#4 #1 or #2 or #3;
#5 MeSH descriptor: [Pancreaticoduodenectomy];
#6 #4 and #5;
#7 MeSH descriptor: [Intraoperative Complications] explode all trees;
#8 MeSH descriptor: [Postoperative Complications] explode all trees;
#9 #7 and #8;
#10 MeSH descriptor: [Learning Curve];
#11 MeSH descriptor: [Education] explode all trees;
#12 #9 or #10 or #11;
#13 #6 and #12.
Inclusion and exclusion criteria
In order to address intraoperative management of complications during and after LPD, we screened systematic reviews for complications of LPD and OPD. For learning curve articles, we aimed to clarify the learning curve expected when implementing LPD. In order to find original data only, we excluded narrative reviews, meta-analysis reviews, and systematic review articles on this topic. For education articles we screened for title and abstract, focusing on articles presenting assessment of, and views on education of LPD.
Limits
Published literature was used as the base of our key concepts and approaches in teaching LPD. Inevitably, this does not capture the vast amount of opinions and experiences on this topic. Risk of bias assessment was not performed since the main aim of the search did not focus primarily on original data.
Results of literature review
Overall, 52 studies were included on basis of title and abstract and the full texts were subsequently read for relevant information in the following categories: complications, education, instrumentation, learning curve. For more detailed information see Figure S1: Search results.
Search results
Fifty-one articles were included on basis of title and abstract and the full texts were read for relevant information in the following categories.
Articles addressing complications
Ojima, Iwahashi et al. 2009; Bausch and Keck 2013; Drymousis, Raptis et al. 2014; Langan, Graham et al. 2014; Lei, Wei et al. 2014; Qin, Qiu et al. 2014; De Rooij, Lu et al. 2016; Chen, Pan et al. 2017; Morikane 2017; Pedziwiatr, Malczak et al. 2017; Shin, Kim et al. 2017; Stauffer, Coppola et al. 2017; Tomita, Chiba et al. 2017.
Articles adressing education
Tait 2002; Gaar 2004; Dixon, Vollmer Jr et al. 2005; Martin and Marion 2007; Alvarado-Bachmann, Choi et al. 2010; Kuroki, Tajima et al. 2010; Yeo 2010; Nakamura, Matsumoto et al. 2012; Gerstenhaber, Grossman et al. 2013; Lee, Han et al. 2013; Lei, Zhifei et al. 2013; Mesleh, Stauffer et al. 2013; Zenoni, Arnoletti et al. 2013; Cho, Yamamoto et al. 2014; Bressan, Edwards et al. 2015; Dhamija, Manish et al. 2016; Hsu, Lin et al. 2016; Kim and Hong 2016; Matsushita, Nakamura et al. 2016; Nappo, Perinel et al. 2016; Wang, Hu et al. 2016; Caruso, Alessandri et al. 2017; Khatkov, Izrailov et al. 2017; Zhang, Liu et al. 2017.
Articles addressing instrumentation
Yang, Tien et al. 2010; Gumbs, Croner et al. 2013; Nissen, Menon et al. 2013.
Articles addressing learning curve
Kim, Ha et al. 2012; Kim, Song et al. 2013; Kuroki, Kitasato et al. 2014; Speicher, Nussbaum et al. 2014; Mendoza, Han et al. 2015; Paniccia, Schulick et al. 2015; Achurra, Sanhueza et al. 2016; Jin, Xu et al. 2016; Mou, Xu et al. 2016; Nagakawa, Hosokawa et al. 2016; Dokmak, Fteriche et al. 2017; Khatkov, Izrailov et al. 2017.
Full text inaccessible
Dhamija, Manish et al. 2016; Wang, Hu et al. 2016.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Abu Hilal M, et al. Worldwide survey on opinions and use of minimally invasive pancreatic resection. HPB (Oxford) 2017;19:190-204. [Crossref] [PubMed]
- Hogg ME, Besselink MG, Clavien PA, et al. Training in Minimally Invasive Pancreatic Resections: a paradigm shift away from “See one, Do one, Teach one”. HPB (Oxford) 2017;19:234-45. [Crossref] [PubMed]
- Dokmak S, Ftériche FS, Aussilhou B, et al. The Largest European Single-Center Experience: 300 Laparoscopic Pancreatic Resections. J Am Coll Surg 2017;225:226-234.e2. [Crossref] [PubMed]
- Speicher PJ, Nussbaum DP, White RR, et al. Defining the Learning Curve for Team-Based Laparoscopic Pancreaticoduodenectomy. Ann Surg Oncol 2014;21:4014-9. [Crossref] [PubMed]
- van der Geest LGM, van Rijssen LB, Molenaar IQ, et al. Volume-outcome relationships in pancreatoduodenectomy for cancer. HPB (Oxford) 2016;18:317-24. [Crossref] [PubMed]
- de Rooij T, van Hilst J, Topal B, et al. Outcomes of a Multicenter Training Program in Laparoscopic Pancreatoduodenectomy (LAELAPS-2). Ann Surg 2019;269:344-50. [Crossref] [PubMed]
- Adam MA, Choudhury K, Dinan MA, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann Surg 2015;262:372-7. [Crossref] [PubMed]
- De Pastena M, van Hilst J, de Rooij T, et al. Laparoscopic Pancreatoduodenectomy With Modified Blumgart Pancreaticojejunostomy. J Vis Exp 2018. [Crossref] [PubMed]
- Subhas G, Mittal VK. Minimally invasive training during surgical residency. Am Surg 2011;77:902-6. [PubMed]
- Birkmeyer JD, Finks JF, O'Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434-42. [Crossref] [PubMed]
- Ahmad G, Gent D, Henderson D, et al. Laparoscopic entry techniques. Cochrane Database Syst Rev 2015;8:CD006583. [PubMed]
- Agresta F, Mazzarolo G, Bedin N. Direct trocar insertion for laparoscopy. JSLS 2012;16:255-9. [Crossref] [PubMed]
- Memon I. Cadaver Dissection Is Obsolete in Medical Training! A Misinterpreted Notion. Med Princ Pract 2018;27:201-10. [Crossref] [PubMed]
- Varas J, Mejía R, Riquelme A, et al. Significant transfer of surgical skills obtained with an advanced laparoscopic training program to a laparoscopic jejunojejunostomy in a live porcine model: feasibility of learning advanced laparoscopy in a general surgery residency. Surg Endosc 2012;26:3486-94. [Crossref] [PubMed]
- Ferrer J, Scott WE, Weegman BP, et al. Pig pancreas anatomy: Implications for pancreas procurement, preservation, and islet isolation. Transplantation 2008;86:1503-10. [Crossref] [PubMed]
- Lee JS, Han JH, Na GH, et al. Laparoscopic pancreaticoduodenectomy assisted by mini-laparotomy. Surg Laparosc Endosc Percutan Tech 2013;23:e98-102. [Crossref] [PubMed]
- DeSouza A, Domajnko B, Park J, et al. Incisional hernia, midline versus low transverse incision: What is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc 2011;25:1031-6. [Crossref] [PubMed]
- Hsu CW, Lin LF, Law MK. Purse-string suture without pancreatic parenchymal stitches in pancreaticojejunostomy during laparoscopic pancreaticoduodenectomy. Surg Pract 2016;20:87-91. [Crossref]
- Nappo G, Perinel J, El Bechwaty M, et al. The Standardization of Pancreatoduodenectomy: Where Are We? Pancreas 2016;45:493-502. [Crossref] [PubMed]
- Pędziwiatr M, Małczak P, Pisarska M, et al. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks Arch Surg 2017;402:841-51. [Crossref] [PubMed]
- de Rooij T, Van Hilst J, Boerma D, et al. Impact of a nationwide training program in minimally invasive distal pancreatectomy (LAELAPS). Ann Surg 2016;264:754-62. [Crossref] [PubMed]
- de Rooij T, van Hilst J, van Santvoort H, et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg 2019;269:2-9. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199-207. [Crossref] [PubMed]
- Hogg ME, Tam V, Zenati M, et al. Mastery-Based Virtual Reality Robotic Simulation Curriculum: The First Step Toward Operative Robotic Proficiency. J Surg Educ 2017;74:477-85. [Crossref] [PubMed]
- Dixon E, Vollmer CM Jr, Bathe O, et al. Training, practice, and referral patterns in hepatobiliary and pancreatic surgery: survey of general surgeons. J Gastrointest Surg 2005;9:109-14. [Crossref] [PubMed]
- Adam MA, Thomas S, Youngwirth L, et al. Defining a Hospital Volume Threshold for Minimally Invasive Pancreaticoduodenectomy in the United States. JAMA Surg 2017;152:336-42. [Crossref] [PubMed]
- Knab LM, Zureikat AH, Zeh HJ 3rd, et al. Towards standardized robotic surgery in gastrointestinal oncology. Langenbecks Arch Surg 2017;402:1003-14. [Crossref] [PubMed]
- Rodriguez HA, Young MT, Jackson HT, et al. Viewer discretion advised: is YouTube a friend or foe in surgical education? Surg Endosc 2018;32:1724-8. [Crossref] [PubMed]
- Achurra P, Lagos A, Avila R, et al. Allowing New Opportunities in Advanced Laparoscopy Training Using a Full High-Definition Training Box. Surg Innov 2017;24:66-71. [Crossref] [PubMed]
- Hogg ME, Zenati M, Novak S, et al. Grading of Surgeon Technical Performance Predicts Postoperative Pancreatic Fistula for Pancreaticoduodenectomy Independent of Patient-related Variables. Ann Surg 2016;264:482-91. [Crossref] [PubMed]
- Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg 2017;104:1443-50. [Crossref] [PubMed]
- Poves I, Burdío F, Morató O, et al. Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy: The PADULAP Randomized Controlled Trial. Ann Surg 2018;268:731-9. [Crossref] [PubMed]
Cite this article as: Zwart MJ, Foppen M, van Hilst J, de Rooij T, Busch OR, Besselink MG. How to teach and train laparoscopic pancreatoduodenectomy. Ann Pancreat Cancer 2019;2:5.