
Partial splenic embolization to alleviate thrombocytopenia in stage III and IV pancreatic ductal adenocarcinoma patients
Introduction
In 2019, it is estimated that there will be 56,770 new cases of pancreas cancer and an estimated 45,750 people will die of this disease, making it the fourth leading cause of cancer related deaths in the United States (1). From 2007–2013 there was a 5-year survival rate of 8.5% in patients with pancreatic cancer (1). Pancreatic ductal adenocarcinomas (PDAC) represent the most common (85%) type of pancreatic neoplasm and metastasis is usually found on presentation (2). Furthermore, primary pancreatic tumors tend to extend into adjacent structures such as the duodenum, the portal vein, or superior mesenteric vessels (2). This local extension to adjacent structures eventually leads to narrowing of major vessels resulting in portal hypertension and thus hypersplenism. The concept of hypersplenism has been studied in liver disease and thought to be a result of portal hypertension causing pooling and sequestration of all corpuscular elements of the blood, predominantly thrombocytes (3).
Partial splenic embolization (PSE), first performed by Spigos et al. in 1979 (4), entails embolization of a portion of the splenic parenchyma utilizing distal embolic agents such as Gelfoam and polyvinyl alcohol particles. PSE has been demonstrated to be a safer and less invasive alternative in comparison to splenectomy and is considered the first-line therapy for thrombocytopenia secondary to hypersplenism in many institutions (5-8). PSE has been used to safely palliate the effects of hypersplenism by reducing the volume of splenic parenchyma using conventional transarterial embolization techniques (9-13). By addressing the effects of hypersplenism, this results in improving platelet counts and thereby allows the continuation of chemotherapy in cancer patients. For example, Kauffman et al. concluded PSE for cancer patients with thrombocytopenia can be performed safely and effectively to increase platelet counts and should be considered an option to facilitate the initiation or resumption of systemic chemotherapy (SC) (14). Another study by Luz et al. concluded that thrombocytopenia from hypersplenism as a result of SC, can be alleviated by PSE thereby allowing the continuation of SC (15).
To date, there have been no studies that have reviewed the impact of PSE on thrombocytopenia in advanced PDAC patients. We conducted a retrospective study examining the impact of PSE on thrombocytopenia in individuals with stage III and IV PDAC.
Methods
We conducted a retrospective chart analysis of patients with stage III or stage IV pancreatic cancer who had undergone PSE between July 5, 2017 and June 4, 2018. Charts were reviewed after approval from HonorHealth Institutional Review Board (IRB). De-identified data including date of diagnosis, cancer stage, location of primary, site of metastasis and date of PSE was collected. Other data extrapolated from patient’s charts included the length of stay in hospital days, platelet counts, symptoms of post-embolization syndrome documented, time in days to resume cancer treatment and which agent administered, and survival information. We also determined maximum coronal diameter of spleen, splenic volume (by utilizing Aquarius iNtuition vers. 4.4 software), and splenic vein patency from pre-embolization cross sectional imaging. The embolic agent used for PSE was recorded. Patient platelet counts were obtained pre-PSE platelet and at one week, one month, and three months.
PSE technique
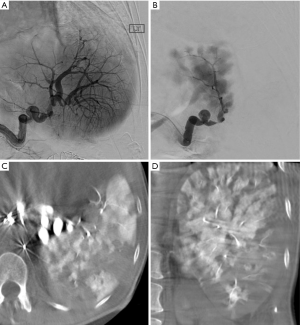
PSEs were performed by four experienced interventional radiologists using standard techniques. All procedures were distal embolizations with selection of embolic agent at the discretion of the radiologist. Agents used were size calibrated non-resorbable microspheres (Embospheres, Merit Medical, South Jordan, UT, USA) measuring 300–500 microns (5 cases), 500–700 microns (4 cases), and one case using Gelfoam slurry. Angiographic technique in all cases was to advance a microcatheter into distal splenic artery branches (to the degree feasible based on anatomy) and perform embolization preferentially targeted for the lower pole of the spleen when possible. The embolization endpoint was roughly 30% devascularization of splenic parenchyma. As shown in Figure 1. It was found that cone beam CT was very helpful intra-procedurally to qualitatively assess the volume of spleen embolized. Patients were admitted for at least overnight observation and pain control. Patients were not given prophylactic vaccinations post-procedure.
Results
Eight patients were identified with either Stage III or IV PDAC and who had undergone PSE. As seen in Table 1, seven of the eight patients were diagnosed with stage IV pancreatic adenocarcinoma with the pancreatic head being the most common primary site (50%). Most common site of metastasis was liver (seven of eight). The median age was 55 years old (range, 39–72 years old). Post-embolization syndrome (PES) was found in five of eight patients with the average number of hospital days after the procedure 1.38 (SD =1.06). The average days to restarting chemotherapy was 24.12 (SD =42.70) and all eight individuals were able to proceed to therapy. As seen in Table 1, most patients resumed FOLFIRINOX, except for one that continued on maintenance metformin. Median overall survival after splenic embolization was 7.22 months (range, 4.67–20.83 months).
Table 1
| Patient # | Cancer stage | Location of primary | Site of metastasis | # Hospital days | Post-embolization syndrome | Time to post-embolization txt (days) | Post-embolization txt | Alive/dead | Overall survival post SPE (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IV | Tail | Liver | 1 | Yes (fever) | 1 | Metformin | Alive | 10.23 |
| 2 | IV | Body | Lung, liver | 1 | Yes (fever, LLQ abd pain) | 11 | Gemcitabine, clinical trial | Deceased | 4.67 |
| 3 | III | Head/ductal | None | 1 | Yes (LUQ abd pain) | 129 | 5FU | Alive | 8.03 |
| 4 | IV | Head | Liver | 1 | No | 8 | FOLFOX | Deceased | 5.23 |
| 5 | IV | Head & Body | Liver | 1 | No | 12 | Clinical trial, maintenance therapy | Deceased | 7.67 |
| 6 | IV | Head | Liver | 1 | Yes (fever, abd pain) | 8 | FOLFIRINOX | Alive | 6.77 |
| 7 | IV | Tail | Stomach, spleen, liver and peritoneum | 1 | No | 5 | FOLFIRINOX | Deceased | 20.83 |
| 8 | IV | Neck/Body | Liver, supraclavicular LN | 4 | Yes (LUQ abd pain) | 19 | Clinical trial | Alive | 4.77 |
txt, treatment; PSE, partial splenc embolization; LN, lymph node; abd, abdomen; LLQ, left lower quadrant; LUQ, left upper quadrant.
Table 2 lists the platelet counts prior to PSE, then at weeks 1, 4, 6, and 12. Mean platelet count pre-splenic embolization was 93.00 (SD =12.59). One-week post-embolization mean platelet count was 147.00 (SD =69.60), four-week was 183.60 (SD =64.93), six-week was 148.40 (SD =40.58), and three-month was 161.00 (SD =79.07). The results of the analysis of variance (ANOVA) were significant, F(4)=3.65, P=0.027, =0.48. Post-hoc analyses revealed significant differences between one-week and four-week post-embolization (P=0.008). Figure 2 provides an overview of the estimated marginal means of platelet counts over the three-month study period.
Table 2
| Patient # | Pre procedure platelet count | 1 week plt count | 4 week Plt count | 6 week Plt count | 12 week Plt count |
|---|---|---|---|---|---|
| 1 | 86 | 84 | 135 | 126 | 104 |
| 2 | 75 | 170 | 206 | 146 | 251 |
| 3 | 67 | 95 | – | 99 | 91 |
| 4 | 97 | 85 | 115 | 162 | 77 |
| 5 | 107 | 252 | 280 | 208 | 238 |
| 6 | 67 | 140 | 83 | 154 | – |
| 7 | 136 | 117 | – | – | 248 |
| 8 | 100 | 144 | 182 | 100 | 135 |
Weeks 4, 6, 12 are +/‒ 1 week. plt, platelet.
Discussion
We performed a retrospective review of eight patients with advanced PDAC. As shown by previous studies, PSE can be considered an accepted and effective alternative to splenectomy for the management of hypersplenism from various pathologies including malignancy directly involving the spleen (9,10,13,16-18). All eight patients in the current study had clinical evidence suggestive of hypersplenism from PDAC. With respect to radiological evidence of hypersplenism, 7/8 met the basic radiologic criterion for splenomegaly (length of spleen greater than 12 cm). A time consuming but more accurate calculation of splenic size is total volume, with 314.5 cm3 having been proposed as the upper limit of normal (19). By this volume definition all 8/8 study patients exhibited splenomegaly (range, 473–886 cm3). Importantly, all patients also had imaging evidence of splenic vein stenosis or occlusion, presumed to cause venous outflow obstruction and congestive hypersplenism. The laboratory evidence included significant thrombocytopenia potentially limiting the administration of SC. Our mean number of days to return to SC for the patients was found to be 24. All, but three of the patients in the study had an elevation in their platelet count within 1 week after undergoing PSE. We also found a statistical significance in elevation of platelet counts between one-week and four-weeks post-embolization (P=0.008).
Metastatic PDAC typically has a median survival of between 11.1 months with FOLFIRINOX, 8.5 months with nab-paclitaxel plus gemcitabine, and 6.8 months with gemcitabine monotherapy (20,21). We found a median overall survival of 7.22 months among our 8 patients with advanced PDAC several of whom had already underwent prior therapeutic regimens. The longest survival was found to be 20.83 months.
With respect to duration of the procedure, we recorded fluoroscopy time and sedation time in our reports. For fluoroscopy time the mean was 19.3 min (range, 8.7–69.4 min), for sedation time the mean was 84 min (range, 55–167 min). This duration can be compared to that of a laparoscopic splenectomy (145.1 min) and open splenectomy (77.3 min) not including sedation time (22). Sedation time is defined in IR as time from first sedation drug administration to time when radiologist leaves the room. We didn't routinely record sedation time until a couple of years ago so that data is lacking for the first two procedures for one of our patients.
Despite the benefits of PSE, it can come with potential complications including PES. Potential complications of PSE include intermittent fever, abdominal pain, nausea, vomiting, splenic abscess, splenic rupture, pneumonia, refractory ascites, pleural effusions, gastrointestinal bleeding and PES (8,11,23,24). PES is defined by low-grade fever, mild abdominal pain, nausea and traditionally treated supportively (5,7,14,25,26). Rarely, severe complications from PSE can arise including sepsis, portal vein thrombosis, and pulmonary infections (24,27). After the retrospective chart analysis, 5/8 or 62.5% of the studied sample met the criteria of PES with the most prominent symptoms of fevers and left sided abdominal pain. In addition to 62.5% of the patient population meeting criteria for PES, one patient (#7 during procedure 11/13/15) experienced a minor complication. There was a small dissection of distal splenic artery due to guide wire manipulation with no detrimental clinical consequence. Although with any procedure, PSE contains its own potential complication including PES, we did not appreciate any major or severe complications in our patient population. There were no other identifiable major or severe complications in our study group.
As seen in Figure 1, we used a rough procedural endpoint of about 30% splenic infarction based on personal experience to balance effectiveness and risk. Furthermore, due to our small sample size, it was difficult to determine if one type of embolic material be superior to others. The embolic agent was selected based on preference of experienced interventional radiologist performing the procedure. We believe total distal splenic embolization using particles could lead to unacceptable post-procedural pain and perhaps increase risk of abscess formation.
Limitations of the study include retrospective analysis and small sample size. Even though our study size consisted of eight patients, they were all diagnosed with hypersplenism from PDAC. Other studies reported that utilized PSE examined a variety of malignancies with very few cases of PDAC (14,15). For example, Kauffman et al. found median overall survival of 9.40 months (95% confidence interval, 8.2–10.7 months) and mean peak platelet value of 293 K/uL among the 28 patients studied (14). The malignancies of their patient population ranged from hepatocellular carcinoma to periampullary/duodenal carcinoma (14). These results were similar is way of substantially increasing platelets values, but differed in there was a longer overall survival rate (9.4 months) compared to our findings (7.22 months).
Despite the small sample size, we were still able to show statistical significant data especially in improving/alleviating thrombocytopenia at 6 and 12 weeks post PSE. Not only did our study demonstrate a statistical significant increase in our platelet counts at the six-week and three-month intervals thereby allowing continuation of SC, we found an overall median survival of 7.22 months. In conclusion, PSE can be considered an effective and tolerable method to alleviate thrombocytopenia in stage III and IV PDAC patients.
PSE should be considered another therapeutic option to alleviate thrombocytopenia secondary to hypersplenism. Validation of its use is warranted in prospective clinical trials.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2019.05.01). E Borazanci: Speaker’s Bureau from Ipsen; Advisory Honoraria from Ipsen, Corcept, Fujifilm; Research funding from Institutional funding with Bristol-Myers Squibb, Pharmacyclics, Idera, Daiichi Sankyo, Minneamrita Therapeutics, Ambry Genetics, Mabvax, Eli Lilly, Samumed. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This report was approved by the Institutional Review Board of HonorHealth medical center (No. 1123527-2). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Vikram R, Balachandran A. Imaging in staging and management of pancreatic ductal adenocarcinoma. Indian J Surg Oncol 2011;2:78-87. [Crossref] [PubMed]
- Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “Hypersplenism” in Thrombocytopenia. J Clin Invest 1966;45:645-57. [Crossref] [PubMed]
- Spigos DG, Jonasson O, Mozes M, et al. Partial splenic embolization in the treatment of hypersplenism. AJR Am J Roentgenol 1979;132:777-82. [Crossref] [PubMed]
- Sibulesky L, Nguyen JH, Paz-Fumagalli R, et al. Treatment modalities for hypersplenism in liver transplant recipients with recurrent hepatitis C. World J Gastroenterol 2009;15:5010. [Crossref] [PubMed]
- He XH, Gu JJ, Li WT, et al. Comparison of total splenic artery embolization and partial splenic embolization for hypersplenism. World J Gastroenterol 2012;18:3138. [Crossref] [PubMed]
- Madoff DC, Denys A, Wallace MJ, et al. Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics 2005;25:S191-211. [Crossref] [PubMed]
- N’Kontchou G, Seror O, Bourcier V, et al. Partial splenic embolization in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol 2005;17:179-84. [Crossref] [PubMed]
- Kimura F, Ito H, Shimizu H, et al. Partial splenic embolization for the treatment of hereditary spherocytosis. AJR Am J Roentgenol 2003;181:1021-4. [Crossref] [PubMed]
- Miyazaki M, Itoh H, Kaiho T, et al. Partial splenic embolization for the treatment of chronic idiopathic thrombocytopenic purpura. AJR Am J Roentgenol 1994;163:123-6. [Crossref] [PubMed]
- Romano M, Giojelli A, Capuano G, et al. Partial splenic embolization in patients with idiopathic portal hypertension. Eur J Radiol 2004;49:268-73. [Crossref] [PubMed]
- Sangro B, Bilbao I, Herrero I, et al. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology 1993;18:309-14. [Crossref] [PubMed]
- Stanley P, Shen TC. Partial embolization of the spleen in patients with thalassemia. J Vasc Interv Radiol 1995;6:137-42. [Crossref] [PubMed]
- Kauffman CR, Mahvash A, Kopetz S, et al. Partial splenic embolization for cancer patients with thrombocytopenia requiring systemic chemotherapy. Cancer 2008;112:2283-8. [Crossref] [PubMed]
- Luz JH, Luz PM, Marchiori E, et al. Partial splenic embolization to permit continuation of systemic chemotherapy. Cancer Med 2016;5:2715-20. [Crossref] [PubMed]
- Athale UH, Kaste SC, Bodner SM, et al. Splenic rupture in children with hematologic malignancies. Cancer 2000;88:480-90. [Crossref] [PubMed]
- Gardner RV, Warrier RP, Loe W, et al. Splenic artery embolization as emergency treatment of splenic rupture in a child with T‐cell acute lymphocytic leukemia having t (8; 14) translocation. Med Pediatr Oncol 2003;41:492-3. [Crossref] [PubMed]
- Kumar S, Diehn F, Gertz M, et al. Splenectomy for immune thrombocytopenic purpura: long-term results and treatment of postsplenectomy relapses. Ann Hematol 2002;81:312-9. [Crossref] [PubMed]
- Bezerra AS, D'Ippolito G, Faintuch S, et al. Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol 2005;184:1510-3. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic Cancer N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Park A, Marcaccio M, Sternbach M, et al. Laparoscopic vs open splenectomy. Arch Surg 1999;134:1263-9. [Crossref] [PubMed]
- Hayashi H, Beppu T, Okabe K, et al. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg 2008;95:744-50. [Crossref] [PubMed]
- Zhu K, Meng X, Qian J, et al. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig. Liver Dis 2009;41:411-6. [Crossref] [PubMed]
- Gonsalves CF, Mitchell EP, Brown DB. Management of hypersplenism by partial splenic embolization with ethylene vinyl alcohol copolymer. AJR Am J Roentgenol 2010;195:1241-4. [Crossref] [PubMed]
- Blackburn H, West S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver Cancer Cancer Nurs 2016;39:E1-8. [Crossref] [PubMed]
- Widlus DM, Moeslein FM, Richard HM III. Evaluation of the Amplatzer vascular plug for proximal splenic artery embolization. J Vasc Interv Radiol 2008;19:652-6. [Crossref] [PubMed]
Cite this article as: Lawson BO, Hultsch R, Caldwell L, Gosselin KP, Jameson G, Borazanci E. Partial splenic embolization to alleviate thrombocytopenia in stage III and IV pancreatic ductal adenocarcinoma patients. Ann Pancreat Cancer 2019;2:9.



