EUS- versus ERCP-guided biliary drainage for malignant biliary obstruction: evidence from randomized controlled studies
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is the most commonly used technique for the management of obstructive jaundice caused by malignant diseases, including cancers which located in the pancreatic head, the duodenal ampulla, or the distal common bile duct (1). Previous evidence has revealed that the overall failure rate of Endoscopic retrograde cholangiopancreatography-guided biliary drainage (ERCP-BD) was about 7% (2). However, in several clinical settings, such as the existence of anatomical variations, ampullary distortion, gastroduodenal obstruction, diverticulum in the duodenum, or preexisting duodenal stents, ERCP was associated with a high rate of failure (5–25%) (1,3). Percutaneous percutaneous transhepatic biliary drainage (PTBD) has been the alternative palliative treatment when ERCP fails. However, previous studies reported that PTBD was associated with high rate of complications, including bleeding, catheter dislocation, bile leakage, recurrent infection, and acute cholangitis (4).
Endoscopic ultrasound-guided biliary drainage (EUS-BD) was firstly introduced by Giovannini et al. in 2001 (5). Substantial evidence has suggested that EUS-BD was safe and feasible, and it can also be an alternative technique after unsuccessful ERCP-BD (6,7). Compared to PTBD, EUS-BD showed better clinical success rate (8), and EUS-BD was also associated with decreased incidence of bleeding and tube dislocation (9). In addition, EUS-BD seemed more cost-effective with fewer unscheduled reintervention than PTBD, and it provided less physical discomfort (8). An recent international multicenter survey also suggested that, when ERCP fails to achieve biliary drainage, most patients preferred EUS-BD over PTBD (10).
Given the safety and feasibility of EUS-BD (8), whether EUS-BD could be the first-line treatment for malignant obstructive jaundice as ERCP-BD remains to be elucidated. The primary aim of the current study is to compare the therapeutic efficacies and complications of EUS-BD and ERCP-BD through a systematic review and meta-analysis.
Methods
Systematic literature search
The current study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses as previously described (11). Published studies were searched in the PubMed, Embase, and Cochrane databases before January 25, 2019. The search terms utilized for EUS- versus ERCP-guided biliary drainage included “endoscopic ultrasound”, “EUS”, “endoscopic retrograde cholangiopancreatography”, “ERCP”, and “biliary”. After removal of duplications, two authors independently screened the identified studies by the titles and abstracts. Then the remaining studies were further assessed for eligibility by reading the full-text. In addition, the references lists of included studies were also screened manually for other potential publications.
Eligibility criteria
Included were studies comparing the therapeutic efficacy and complications of patients who underwent EUS-BD or ERCP-BD, and the studies should meet the following criteria: (I) randomized clinical trials (RCTs); (II) biliary obstruction caused by malignant disease; (III) comparable data of EUS-BD and ERCP-BD was available, and (IV) reported at least one outcome of interest as mentioned blow. Excluded were articles reporting the biliary obstruction caused by stones or other benign diseases, articles without records of the therapeutic efficacy or complications, meta-analysis or review articles, and articles in other languages other than English.
Assessment of methodological quality
According to the modified Jadad scoring criteria (12,13), the methodological quality of all the included randomized studies was assessed by 2 authors independently. Every included study was evaluated in the following four aspects: (I) randomization, (II) concealment of allocation, (III) double blinding, (IV) withdrawals and dropouts. The maximum score for a randomized study is 7 according the criteria. Randomized studies with modified Jadad score ranged from 1 to 3 are considered as low quality. Studies with the modified Jadad score ranged from 4 to 7 are considered as high quality.
Data extraction
The data extracted from the included studies included the following variables, study characteristics (first author, publication time/study period, the country of study, study design, surgical procedure, and sample size), the baseline characteristics of patients (age, gender, pathological diagnosis, baseline total bilirubin, common bile duct diameter), The therapeutic efficacy (technical success rates, clinical success rates, procedure time, stent patency), and procedure-associated complications (mild/moderate/severe adverse events, procedure-related pancreatitis, procedure-related cholangitis, and reintervention rates). We collected these outcomes as defined in original studies. The extracted data were re-checked by two authors independently.
Statistical analysis
We performed the meta-analysis according to the Cochrane guidelines, and the data were reviewed and analyzed using Review Manager 5.3 software (The Cochrane Collaboration, Oxford, UK). In the current study, categorical variables were presented as percentages or frequencies, and continuous variables were presented as mean with standard deviations (SD). Those data which were presented as median with ranges were converted to the form of mean and SD according to the statistical algorithms described by Hozo et al. (14). For statistical analysis, we used the I2 to detect the heterogeneity among the included studies. Fixed-effects model (FEM) was used if there was no significant heterogeneity (I2<50% or I2=50%), and random-effects model (REM) was used in the setting of considerable heterogeneity (I2>50%). The Mantel-Haenszel method was used to compare the clinical data of EUS-BD and ERCP-BD. In the forest plot, we presented the results with odds ratio (OR) and 95% confidence intervals (CI). P<0.05 was considered as significant between EUS-BD and ERCP-BD.
Results
Search results
The study selection flow diagram for the current study is summarized in Figure 1. A total of 821 studies were identified. After removal of duplications and screening of the titles and abstracts, we further screened the remaining 31 studies by reading the full text. Additionally, 28 studies were excluded for the reasons that there were no comparable data of EUS-BD and ERCP-BD, the pathological diagnosis were not malignant diseases, or studies without randomization. Finally, we identified three randomized clinical studies reporting the therapeutic efficacies and complications of patients who underwent EUS-BD or ERCP-BD because of malignancies, and a total of 220 patients (EUS-BD/ERCP-BD, 111/109) with malignant biliary obstruction were included. The basic characteristics of the included studies were summarized in Table 1.
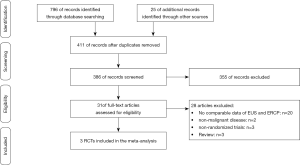
Table 1
| Author | Year (study period) | Country | Diagnosis | Study design | Procedure/cases | |
|---|---|---|---|---|---|---|
| EUS-BD | ERCP-BD | |||||
| Paik et al. (15) | 2018/(2015–2017) | Korea | Malignant | RCT, M | 64 | 61 |
| Park et al. (16) | 2018/(NA) | Korea | Malignant | RCT, S | 14 | 14 |
| Bang et al. (17) | 2018/(2016–2017) | USA | Pancreatic | RCT, S | 33 | 34 |
EUS, endoscopic ultrasound; ERCP, endoscopic retrograde cholangiopancreatography; RCT, randomized controlled trial; M, multicenter; S, single-center; NA, not available.
Methodological quality
We used the modified Jadad score to assess the methodological quality according to the criteria as previously described (12,13). The two articles reported by Paik et al. and Bang et al. were of high quality (15,16), and both articles were scored 7. The article reported by Park et al. earned a score of 3, which was considered to be of low quality (17). Description of the methods of allocation concealment and blinding were lacking in the study reported by Park et al. The details of the assessment are shown in Table 2.
Table 2
| Clinical trails | Randomization | Concealment of allocation | Double blinding | Withdrawals and dropouts | Jadad score* |
|---|---|---|---|---|---|
| Paik et al. (15) | 2 | 2 | 2 | 1 | 7 |
| Park et al. (16) | 2 | 0 | 0 | 1 | 3 |
| Bang et al. (17) | 2 | 2 | 2 | 1 | 7 |
*, methodological quality of meditative movements studies reviewed using modified Jadad scoring criteria. Total score is 7. Score 1 to 3 considered as low quality; score 4 to 7 considered as high quality.
Patient characteristics
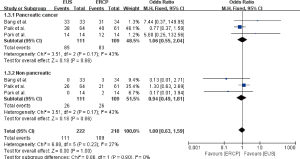
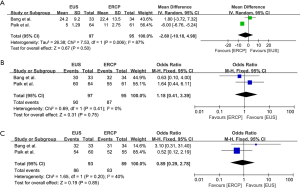
The patient characteristics reported by the included studies were summarized in Table 3. Patients underwent EUS-BD or ERCP-BD did not differ in age (OR =−1.57, 95% CI: −4.52 to 1.38, P=0.30), gender (OR =1.22, 95% CI: 0.43 to 3.47, P=0.72), baseline total bilirubin (OR =0.36, 95% CI: −1.45 to 2.17, P=0.70), and common bile duct (CBD) diameter (OR =0.77, 95% CI: −0.28 to 1.82, P=0.15) (Figure 2). Of the 220 patients included, a total of 168 (76%) patients were diagnosed as pancreatic cancer, 52 patients (24%) were diagnosed as non-pancreatic malignancies, which included 11 cases of cholangiocarcinoma, 8 cases of gallbladder cancer, 8 cases of Ampulla of Vater cancer, 6 cases of stomach cancer, 3 cases of duodenal cancer, 1 case of hepatocellular carcinoma and 15 cases of other types of malignancies (15-17). The meta-analysis showed that the diagnosis did not differ between the EUS-BD and ERCP-BD groups (OR =1.06, 95% CI: 0.55 to 2.04, P=0.86) (Figure 3).
Table 3
| Author | Procedure | Age (year) | Gender (male): n (%) | Diagnosis | Total bilirubin (mg/dL) | CBD diameter (mm) | |
|---|---|---|---|---|---|---|---|
| Pancreatic cancer | Non-pancreatic | ||||||
| Paik et al. (15) | EUS-BD | 64.8±12.5 | 41 (66.1%) | 38/64 | 26/64 | 8.3±7.2 | 15.7±4.0 |
| ERCP-BD | 68.4±10.5 | 26 (42.6%) | 40/61 | 21/61 | 7.7±6.4 | 15.0±3.9 | |
| Park et al. (16) | EUS-BD | 66.8±8.0 | 9 (62.3%) | 14/14 | 0/14 | 7.5 (4.8, 14.0)* | NA |
| ERCP-BD | 65.4±9.3 | 8 (57.1%) | 12/14 | 2/14 | 9.9 (7.5, 20.4)* | NA | |
| Bang et al. (17) | EUS-BD | 69.4±12.6 | 17 (51.5%) | 33/33 | 0/33 | 12.5±6.3 | 13.3±3.5 |
| ERCP-BD | 69.2±11.6 | 23 (67.6%) | 31/34 | 3/34 | 12.1±5.9 | 12.5±3.7 | |
*, median and IQR. CBD, common bile duct; EUS-BD, endoscopic ultrasound-guided biliary drainage; ERCP-BD, endoscopic retrograde cholangiopancreatography-guided biliary drainage; NA, not available.

Therapeutic efficacy
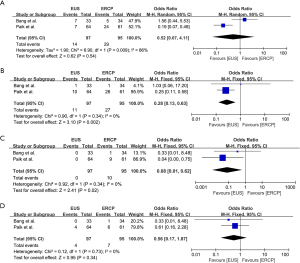
The technical success rate, clinical success rate and procedure time of EUS-BD or ERCP-BD were recorded in all of the three randomized clinical studies (15-17) (Table 4). Meta-analysis of long-term survival was showed in Figure 4. The technical success rate (OR =1.02, 95% CI: 0.38 to 2.73, P=0.97), clinical success rate (OR =1.04, 95% CI: 0.36 to 2.97, P=0.94), and procedure time (OR =−0.24, 95% CI: −8.28 to 7.79, P=0.95) were not significantly different between EUS-BD and ERCP-BD groups. The stent patency was recorded in two studies. Compared to ERCP-BD, EUS-BD showed similar 3-month stent patency (OR =1.67, 95% CI: 0.07 to 41.71, P=0.75) and 6-month stent patency (OR =2.87, 95% CI: 0.55 to 14.80, P=0.21). Surprisingly, the 12-month stent patency (OR =3.23, 95% CI: 2.06 to 5.07, P<0.0001) was significantly higher in EUS-BD group (Figure 4).
Table 4
| Author | Procedure | Technical success rates | Clinical success rates | Procedure time (min) | Stent patency | ||
|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | |||||
| Paik et al. (15) | EUS-BD | 93.8% (60/64) | 90.0% (54/60) | 5 (3–12)* | 62 (96.9%) | 54 (85.0%) | 40 (62.5%) |
| ERCP-BD | 90.2% (55/61) | 94.5% (52/55) | 11 (7–18)* | 49 (80.3%) | 30 (49.0%) | 22 (36.1%) | |
| Park et al. (16) | EUS-BD | 92.8% (13/14) | 100% (13/13) | 43.0±24.0 | 11 (78.6%) | 11 (78.6%) | 11 (78.6%) |
| ERCP-BD | 100% (14/14) | 92.8% (13/14) | 31.0±21.0 | 13 (92.8%) | 11 (78.6%) | 9 (64.3%) | |
| Bang et al. (17) | EUS-BD | 90.9% (30/33) | 97.0% (32/33) | 24.2±9.2 | NA | NA | NA |
| ERCP-BD | 94.1% (32/34) | 91.2% (31/34) | 22.4±13.5 | NA | NA | NA | |
*, median and IQR. EUS-BD, endoscopic ultrasound-guided biliary drainage; ERCP-BD, endoscopic retrograde cholangiopancreatography-guided biliary drainage; NA, not available.
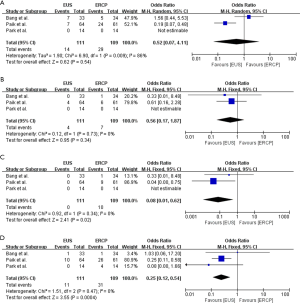
Procedure-associated complications
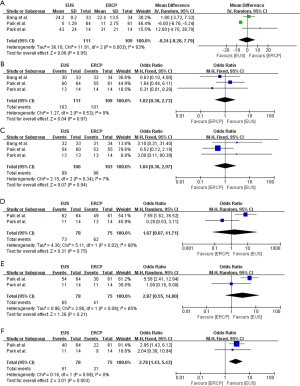
The procedure-associated complications reported by the included studies were summarized in Table 5. Severe adverse events did not occur in any of the three studies included, and all of the recorded adverse events were of mild and moderate (Table 5). The results showed that there was no significant difference between the EUS-BD and ERCP-BD groups regarding to the overall adverse events (OR =0.52, 95% CI: 0.07 to 4.11, P=0.54) (Figure 5A). The incidence of procedure-associated cholangitis was equivalent between EUS-BD and ERCP-BD (OR =0.56, 95% CI: 0.17 to 1.87, P=0.34) (Figure 5B). In the ERCP-BD group, 10 of the included patients occurred procedure-associated pancreatitis. No pancreatitis occurred in the EUS-BD group, and the results showed significant difference (OR =0.08, 95% CI: 0.01 to 0.62, P=0.02) (Figure 5C). In addition, we also revealed that the reintervention rate in the EUS-BD group was significantly lower than that in ERCP-BD group (OR =0.25, 95% CI: 0.12 to 0.54, P=0.0004) (Figure 5D).
Table 5
| Author | Procedure | Adverse events | Pancreatitis | Cholangitis | Reintervention | |||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Overall | |||||
| Paik et al. (15) | EUS-BD | 4 (6.3%) | 3 (4.7%) | 0 (0.0%) | 7 (11.0%) | 0 (0.0%) | 4 (6.3%) | 10 (15.6%) |
| ERCP-BD | 16 (26.2%) | 8 (13.1%) | 0 (0.0%) | 24 (39.3%) | 9 (14.8%) | 6 (9.8%) | 26 (42.6%) | |
| Park et al. (16) | EUS-BD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| ERCP-BD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (30.8%) | |
| Bang et al. (17) | EUS-BD | 5 (15.2%) | 2 (6.1%) | 0 (0.0%) | 7 (21.2%) | 0 (0.0%) | 0 (0.0%) | 1 (3.0%) |
| ERCP-BD | 3 (8.8%) | 2 (5.9%) | 0 (0.0%) | 5 (14.7%) | 1 (2.9%) | 1 (2.9%) | 1 (2.9%) | |
EUS-BD, endoscopic ultrasound-guided biliary drainage; ERCP-BD, endoscopic retrograde cholangiopancreatography-guided biliary drainage.
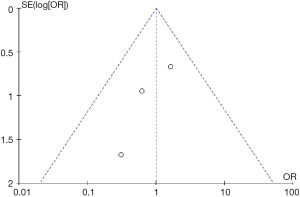
Given the result that the article reported by Park et al. was considered to be of low quality according to the Jadad score (Table 2), we performed a sub-analysis in which the study conducted by Park et al. was excluded. The sub-analysis revealed that technical success rate (OR =1.18, 95% CI: 0.41 to 3.39, P=0.75), clinical success rate (OR =0.89 95% CI: 0.29 to 2.78, P=0.85), procedure time (OR =−2.60, 95% CI: −10.18 to 4.98, P=0.50) were also equivalent between ERCP-BD and EUS-BD groups (Figure S1). In addition, the sub-analysis showed that the overall adverse events (OR =0.52, 95% CI: 0.07 to 4.11, P=0.54) and procedure-related cholangitis (OR =0.56, 95% CI: 0.17 to 1.87, P=0.34) were also not significantly different (Figure S2). However, the reintervention rate (OR =0.28, 95% CI: 0.13 to 0.63, P=0.002), procedure-related pancreatitis (OR =0.08, 95% CI: 0.01 to 0.62, P=0.02) were significant decreased in the EUS-BD group (Figure S2). In the current study, funnel plot of the technical success rate was used to detect the publication bias. Visual inspection of the funnel plot presented symmetry, indicating no obvious publication bias in the current study (Figure 6).
Discussion
With a success rate more than 95% in experienced hands, ERCP-BD has been the standard treatment for biliary decompression in patients with malignant biliary obstruction when the patients were not suitable for curative surgery (18,19). ERCP also has some disadvantages, such as postinterventional pancreatitis, traumatic injury of the main pancreatic duct, and stent occlusion caused by ingrowth of the tumor (20,21). Paik et al. reported that ERCP-BD achieved an overall technical success rate of 90.2%. However, in the presence of duodenal invasion, the success rate of ERCP was reduced to 66.7% (16). A previous study reported only a 56% of technical success rate in patients with an indwelling gastroduodenal stent (22). EUS-BD has been used as an alternative treatment in the past when ERCP-BD fails. A wealth of evidence has also revealed the safety and feasibility of EUS-BD in the management of malignant biliary obstruction (23-25). The current study aimed to compare the therapeutic efficacies and procedure-associated complications of EUS-BD and ERCP as the first treatment option.
Previous retrospective study conducted by Kanno et al. showed that in cases wherein ERCP was impossible or ineffective, EUS-BD still achieved a technically success rate of 98% and a clinical success rate of 93% (6). Another study reported that in patients with malignant biliary obstruction and complicated with ascites, EUS-BD was also technically feasible (7). Several studies have compared the therapeutic efficacies of EUS-BD and ERCP-BD (22,26,27). Evidence from a retrospective case control study analyzed 208 patients who were diagnosed as malignant distal common bile duct obstruction and required for placement of self-expandable metal stent (SEMS), and the authors reported that the short-term outcome of EUS-BD is comparable to that of ERCP (26). However, one of the drawbacks of this retrospective study was that EUS-BD was performed after the failure of ERCP-BD, rather than as a first-line treatment. Another retrospective study conducted by Kawakubo et al. reported a promising efficacy of EUS-BD as a first-line treatment for patients with distal malignant biliary obstruction, and EUS-BD was associated with shorter procedure time and no risk of pancreatitis (27). The retrospective nature of these cohort studies was associated with inevitable selection bias, and several variables such as gender and stent diameter could impact the therapeutic effects (20). Therefore, only RCTs were included in the current meta-analysis to compare the therapeutic efficacies and complications of EUS-BD and ERCP-BD.
In the current study, we included the currently published three RCTs to compare the therapeutic effects of EUS-BD and ERCP-BD as the first choice of treatment. The results suggested that the therapeutic efficacy of EUS-BD was equivalent to that of ERCP-BD, and the technical and clinical success rates were similar between the two groups. Regarding to the incidence of procedure-related complications, EUS-BD was associated with decreased reintervention and procedure-associated pancreatitis. In addition, the frequency of stent patency at twelve months was also high in the EUS-BD group (Table 6). Our results were consistent with previous evidence that EUS-BD could be the first option of treatment for malignant biliary obstruction. In addition, the study by Paik and colleagues suggested that more preserved quality of life (QOL) were observed in the EUS-BD group.
Table 6
| Result | Study number | Sample size (EUS/ERCP) | Heterogeneity (P, I2) | Model | WMD/OR (95% CI) | P |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age | 3 | 111/109 | 7% | F | −1.57 (−4.52, 1.38) | 0.3000 |
| Gender | 3 | 111/109 | 68% | R | 1.22 (0.43, 3.47) | 0.7200 |
| Diagnosis | 3 | 111/109 | 43% | F | 1.06 (0.55, 2.04) | 0.8600 |
| Total bilirubin | 3 | 111/109 | 0% | F | 0.36 (−1.45, 2.17) | 0.7000 |
| CBD diameter | 2 | 97/95 | 0% | F | 0.77 (−0.28, 1.82) | 0.1500 |
| Therapeutic efficacy | ||||||
| Technical success rate | 3 | 111/109 | 0% | F | 1.02 (0.38, 2.73) | 0.9700 |
| Clinical success rate | 3 | 106/103 | 7% | F | 1.04 (0.36, 2.97) | 0.9400 |
| Procedure time | 3 | 111/109 | 83% | R | −0.24 (−8.28, 7.79) | 0.9500 |
| Stent patency (3 months) | 2 | 78/75 | 80% | R | 1.67 (0.07, 41.71) | 0.7500 |
| Stent patency (6 months) | 2 | 78/75 | 65% | R | 2.87 (0.55, 14.80) | 0.2100 |
| Stent patency (12 months) | 2 | 78/75 | 0% | F | 3.23 (2.06, 5.07) | <0.0001 |
| Complication rate | ||||||
| Overall adverse events | 3 | 111/109 | 86% | R | 0.52 (0.07, 4.11) | 0.5400 |
| Pancreatitis | 3 | 111/109 | 0% | F | 0.08 (0.01, 0.62) | 0.0200 |
| Cholangitis | 3 | 111/109 | 0% | F | 0.56 (0.17, 1.87) | 0.3400 |
| Reintervention rate | 3 | 111/109 | 0% | F | 0.25 (0.12, 0.54) | 0.0004 |
EUS, endoscopic ultrasound-guided; CBD, common bile duct; F, fixed-effects model; R, random-effects model.
This study also has several limitations. One is that the number of patients included is relatively small. So far there are only three RCTs reported the comparison of EUS-BD and ERCP-BD for malignant disease. Another factor that could affect the outcomes of is the procedure learning curve, which was not described in detail in the included studies. The difference of the learning curves between the three included studies was reflected by the fact that the reintervention rate varied a lot between different studies. In addition, in the three RCTs, there was heterogeneity in the use of stents as the stents used in different studies come from different manufacturers.
Conclusions
In the current study, we revealed that, for the treatment of malignant obstructive jaundice, the therapeutic efficacies of EUS-BD were equivalent to that of ERCP-BD, given the result that the technical success rate, clinical success rate and procedure time were similar between the two groups. EUS seemed superior regarding to the 12-month stent patency, procedure-related pancreatitis and reintervention rate. Our results suggested that EUS-BD, like ERCP-BD, could be the first-line technique for the management of malignant bilairy obstruction. Due to the small number of cases included in this study, large sample RCT is still needed to validate the result.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81802623), Zhejiang Province Key Science and Technology (2015C03049), Zhejiang Province Major Science and Technology (2015C03G1360047), Zhejiang Province Medical and Health Platform support (2015DTA010).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Min Li) for the series “Science on Pancreatic Cancer” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2019.08.01). The series “Science on Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baars JE, Kaffes AJ, Saxena P. EUS-guided biliary drainage: A comprehensive review of the literature. Endosc Ultrasound 2018;7:4-9. [Crossref] [PubMed]
- Coté GA, Singh S, Bucksot LG, et al. Association between volume of endoscopic retrograde cholangiopancreatography at an academic medical center and use of pancreatobiliary therapy. Clin Gastroenterol Hepatol 2012;10:920-4. [Crossref] [PubMed]
- Saxena P, Khashab MA. First-line EUS-guided biliary drainage or ERCP in patients with biliary obstruction and in situ duodenal stent? Gastrointest Endosc 2018;88:76-8. [Crossref] [PubMed]
- Nennstiel S, Weber A, Frick G, et al. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol 2015;49:764-70. [Crossref] [PubMed]
- Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001;33:898-900. [Crossref] [PubMed]
- Kanno Y, Koshita S, Ogawa T, et al. EUS-Guided Biliary Drainage for Unresectable Malignant Biliary Obstruction: 10-Year Experience of 99 Cases at a Single Center. J Gastrointest Cancer 2019;50:469-77. [Crossref] [PubMed]
- Alvarez-Sánchez MV, Luna OB, Oria I, et al. Feasibility and Safety of Endoscopic Ultrasound-Guided Biliary Drainage (EUS-BD) for Malignant Biliary Obstruction Associated with Ascites: Results of a Pilot Study. J Gastrointest Surg 2018;22:1213-20. [Crossref] [PubMed]
- Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc 2017;85:904-14. [Crossref] [PubMed]
- Duan F, Cui L, Bai Y, et al. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: a systematic review and meta-analysis. Cancer Imaging 2017;17:27. [Crossref] [PubMed]
- Nam K, Kim DU, Lee TH, et al. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound 2018;7:48-55. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008;88:156-75. [Crossref] [PubMed]
- Oremus M, Wolfson C, Perrault A, et al. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord 2001;12:232-6. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Paik WH, Lee TH, Park DH, et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol 2018;113:987-97. [Crossref] [PubMed]
- Park JK, Woo YS, Noh DH, et al. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc 2018;88:277-82. [Crossref] [PubMed]
- Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc 2018;88:9-17. [Crossref] [PubMed]
- Geraci G, Sciume C, Pisello F, et al. Endoscopic palliation of obstructive jaundice caused by inoperable pancreatic cancer: personal experience. Ann Ital Chir 2005;76:473-6. [PubMed]
- Das A, Sivak MV Jr. Endoscopic palliation for inoperable pancreatic cancer. Cancer Control 2000;7:452-7. [Crossref] [PubMed]
- Hashimoto S, Ito K, Koshida S, et al. Risk Factors for Post-Endoscopic Retrograde Cholangiopancreatography (ERCP) Pancreatitis and Stent Dysfunction after Preoperative Biliary Drainage in Patients with Malignant Biliary Stricture. Intern Med 2016;55:2529-36. [Crossref] [PubMed]
- Matsubayashi H, Fukutomi A, Kanemoto H, et al. Risk of pancreatitis after endoscopic retrograde cholangiopancreatography and endoscopic biliary drainage. HPB (Oxford) 2009;11:222-8. [Crossref] [PubMed]
- Yamao K, Kitano M, Takenaka M, et al. Outcomes of endoscopic biliary drainage in pancreatic cancer patients with an indwelling gastroduodenal stent: a multicenter cohort study in West Japan. Gastrointest Endosc 2018;88:66-75 e2.
- Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP 2007;8:438-43. [PubMed]
- Artifon EL, Okawa L, Takada J, et al. EUS-guided choledochoantrostomy: an alternative for biliary drainage in unresectable pancreatic cancer with duodenal invasion. Gastrointest Endosc 2011;73:1317-20. [Crossref] [PubMed]
- Artifon EL, Takada J, Okawa L, et al. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP 2010;11:597-600. [PubMed]
- Dhir V, Itoi T, Khashab MA, et al. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc 2015;81:913-23. [Crossref] [PubMed]
- Kawakubo K, Kawakami H, Kuwatani M, et al. Endoscopic ultrasound-guided choledochoduodenostomy vs. transpapillary stenting for distal biliary obstruction. Endoscopy 2016;48:164-9. [PubMed]
Cite this article as: Chen K, Chen Z, Jin W, Zhu Q, Lu C, Wang Y, Zhou Y, Mou Y. EUS- versus ERCP-guided biliary drainage for malignant biliary obstruction: evidence from randomized controlled studies. Ann Pancreat Cancer 2019;2:16.