
Adding combination immunotherapy consisting of cancer vaccine, anti-PD-1 and anti-CSF1R antibodies to gemcitabine improves anti-tumor efficacy in murine model of pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDA) responds poorly to immune checkpoint blockade. One way to elicit an immunogenic response to immune checkpoint inhibition may be chemotherapy, as many preclinical and clinical studies have demonstrated improved anti-tumor responses and survival with combination chemotherapy/immunotherapy regimens (1-4). Immunogenic cell death caused by chemotherapy can prime antigen-presenting cells and lead to anti-tumor cytotoxic T-cell responses (5-8). Dendritic cells engulf the antigens released from apoptotic cells and prime CD8+ T-cells via major histocompatibility class I molecules (9). Chemotherapy also increases the expression of death receptors and Fas ligand on tumors, both of which are used by T-cells to induce tumor cell death (10).
Gemcitabine is a commonly used chemotherapy for the treatment of PDA with proven clinical benefit, and is also known for its immune modulating anti-tumor effects (11-13). Chemotherapy-induced apoptosis by gemcitabine dramatically increases the number of lymphocytes involved with antigen-presentation in an in vivo tumor model (14). Gemcitabine has also been shown to decrease myeloid suppressor cells in tumors, enhance cross-presentation of tumor antigens, improve the efficacy of cancer vaccines and augment anti-tumor immunity (15-18). However, the cytotoxic effect of gemcitabine on PDA is limited, and its immunomodulatory role has not been studied in vivo in syngeneic orthotopic murine models of PDA.
Like many treatment modalities that prime the tumor microenvironment (TME), chemotherapy also upregulates programmed death-ligand 1 (PD-L1) expression in tumors, and over-expression of PD-L1 or programmed death-1 (PD-1) is associated with response to immune checkpoint blockade in many cancers (1,2). In our previous studies, we demonstrate that the granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cancer vaccine, GVAX, also increases the expression of PD-1/PD-L1 in pancreatic cancer patients, and combination treatment with GVAX and PD-1/PD-L1 inhibition improves survival in murine models of metastatic PDA (19,20).
We also found that higher myeloid cell infiltration is associated with poorer survival in patients who receive GVAX (21). Myeloid marker, colony-stimulating factor-1 (CSF-1), and its receptor, CSF-1R, are critical for the differentiation, migration and survival of myeloid cells (22). Mitchem et al. demonstrate that the inhibition of CSF-1R decreases tumor-associated macrophages (TAMs) and monocytic-myeloid-derived suppressor cells (M-MDSCs), and that the addition of anti-CSF-1R antibody (αCSF-1R) to gemcitabine further improves the anti-tumor response (23). αCSF-1R therapy increases PD-1 expression, and the addition of checkpoint inhibition and gemcitabine to αCSF-1R therapy improves anti-tumor activity and decreases the number of TAMs, M-MDSCs and granulocytic (PMN)-MDSCs (G-MDSCs) (24). The addition of anti-PD-1 antibody (αPD-1) counteracts the PD-1/PD-L1 pathway that is upregulated with αCSF-1R or GVAX therapy (20,24). Finally, the combination therapy of GVAX, αPD-1 and αCSF-1R improves survival in a murine model of liver-metastatic PDA, as well as increases the infiltration of cytotoxic CD8+ T-cells into the tumor (25).
To explore the immunomodulatory effects of gemcitabine in vivo, we investigated if the addition of GVAX, αPD-1 and αCSF-1R to this chemotherapy improved survival in a murine model of metastatic PDA, and explored the tumor immune effects of this chemo-immunotherapy regimen.
Methods
Cell lines and media
KPC is a well-established PDA cell line that was generated in our lab (26) from transgenic mice using the Cre/lox system in a C57BL/6 genetic background with pancreas-specific expression of the oncogenes KRAS and p53 (27). KPC cells were cultured in RMPI 1640 media (Gibco) supplemented with 1% penicillin/streptomycin (pen/strep, Gibco), 10% heat-inactivated fetal bovine serum (HI-FBS, Benchmark), 1% Minimum Essential Medium-Non-Essential Amino Acids (MEM-NEAA, Life Technologies), 1% L-glutamine (Life Technologies) and 1% sodium pyruvate (Sigma). B78H1 is a major histocompatibility complex class I-negative murine fibroblast cell line engineered to secrete GM-CSF. B78H1 cells were cultured in RMPI 1,640 media supplemented with 1% pen/strep, 10% HI-FBS and 0.5% L-glutamine. Both KPC cells and B78H1 cells were maintained at 37 °C in a 5% CO2 humidified incubator. Tumor-infiltrating immune cells harvested from mice were processed in T-cell media, which consisted of RPMI 1640 media supplemented with 1% pen/strep, 10% HI-FBS, 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, Life Technologies), 1% MEM-NEAA, 0.5% L-glutamine and 0.05% 2-mercaptoethanol (Sigma). For isolation of CD8+ T-cells, harvested tumor-infiltrating immune cells were suspended in Isolation Buffer, which consisted of PBS (Ca2+ and Mg+ free, pH 7.4; Gibco), 0.1% weight/volume bovine serum albumin (BSA, Sigma) and 2 mM ethylenediaminetetraacetic acid (EDTA, Gibco).
Mice tumor inoculation
Seven- to eight-week-old C57Bl6 mice were purchased from Jackson Laboratories, and cared for in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. When the mice were 9- to 10-week-old, they were inoculated with KPC tumor cells via the hemi-spleen technique to generate liver metastasis (day 0) as previously published (28). Briefly, after the mouse was anesthetized, the spleen was eviscerated, clipped and cut in half. One half of the spleen was placed back into the abdominal cavity. Into the other half of the spleen, 2×105 KPC cells in 100 µL of PBS were gently injected and then flushed with 150 µL of PBS. The hemi-spleen that was used to inoculate the tumor cells was then removed to prevent residual tumor cells from remaining external to the liver. The peritoneum and the skin were sutured closed.
Treatment regimen
In the treatment regimens that included both immunotherapy and gemcitabine, the two classes of therapy were administered sequentially, with gemcitabine given first, followed by immunotherapy (i.e., αPD-1, αCSF-1R and GVAX). Treatment started four days after tumor inoculation, with gemcitabine (Hospira) administered at 1 mg (50 mg/kg) intraperitoneally on days 4 and 7 (where day 0 was the day of tumor inoculation). On day 11, 4 days after the second dose of gemcitabine was given, 100 µg (5 mg/kg) of anti-mouse αPD-1 (clone 4H2, IgG1, Bristol-Myers-Squibb) and 1.5 mg (75 mg/kg) of rat anti-mouse αCSF-1R (clone AFS98, IgG2a, BioXcell) were administered intraperitoneally, and murine GVAX vaccine was administered subcutaneously. αPD-1 and αCSF-1R were continued twice a week for 3 weeks, and GVAX once a week for 3 weeks. In the control groups that contain only gemcitabine, it was given twice a week, starting day 4, for 3 to 4 weeks depending on the experiment.
To prepare the GVAX vaccine, KPC and B78H1 cells in PBS were combined at a 1:1 ratio of cells to a total combined cell concentration of 20×106 cells/mL, and irradiated at 50 Gy. The vaccine was then administered subcutaneously in three locations (one of the upper limbs and bilateral flanks) at 100 µL per injection (1×106 KPC and 1×106 B78H1 cells per injection; 6×106 cells injected total per dose per mouse).
Survival studies
There were ten mice per treatment group for each experiment. The mice were monitored at least twice a day, and survival was censored at 120 days. Mice were euthanized if they displayed signs of distress, such as hunched posture and lethargy, and were determined to have reached the “survival” endpoint.
Harvesting tumor-infiltrating immune cells
Eighteen days after tumor inoculation, which was 3 days after the last dose of therapy, the mice were sacrificed and the metastatic liver tumors were harvested. The livers were mechanically pressed through a 100- and 40-µm nylon filter (Falcon) sequentially in T-cell media, and the tissue was resuspended in 25 mL of T-cell media and centrifuged at 1,500 rpm for 5 minutes. After the supernatant was aspirated, 4 mL of ACK lysing buffer (Quality Biological) was added to the cell pellets. After 2 minutes in the ACK buffer, the lysing was quenched by adding 50 mL of T-cell media. The samples were centrifuged again at 1,500 rpm for 5 minutes, and the supernatant was aspirated. The cell pellets were resuspended in 6 mL of 80% Percoll (GE Healthcare), over which 6 mL of 40% Percoll was added. The samples were then centrifuged without braking at 3,200 rpm for 25 minutes. The leukocyte layer was collected and resuspended in 50 mL of T-cell media, washed twice and resuspended in either PBS for staining for flow cytometry or Isolation Buffer for isolation of CD8+ T-cells. There were four mice per treatment group for each experiment. The cells from each mouse were not pooled, and were kept separate.
Isolating and activating tumor-infiltrating CD8+ T-cells
The isolated tumor-infiltrating immune cells were re-suspended at 100×106 cells/mL in Isolation Buffer, and negative isolation technique was used to enrich for CD8+ T-cells as per the manufacturer’s protocol (Dynabeads Untouched Mouse CD8 cells, Life Technologies). The samples enriched for CD8+ T-cells were mixed with CD3/CD28 stimulation beads (Dynabeads Mouse T-activator CD3/CD28, Life Technologies) at 1:1 ratio as per the manufacturer’s protocol. The T-cells and stimulation beads were incubated at 37 °C in a 5% CO2 humidified incubator for 6 hours before GolgiPlug (BD Biosciences) was added at 1:1,000 volume ratio. The cells were then incubated for 5 more hours at 37 °C in 5% CO2. After 11 hours of total incubation time, a magnetic rack was used to remove the beads, and the cell suspensions were centrifuged for 1,500 rpm for 5 minutes in 4 °C, and washed twice with PBS. There were four mice per treatment group for each experiment. The cells from each mouse were not pooled, and were kept separate.
Cell surface and intracellular staining for interferon-γ and flow cytometry
The cells were resuspended in 100 µL of PBS and placed in a 96-well v-bottom plate (Corning). The cells were stained with Live-Dead Aqua (Invitrogen) for 30 minutes on ice, washed twice with PBS and blocked with rat anti-mouse Fc antibody (CD16/CD32, clone 2.4G2, BD Biosciences) in FACs buffer for 10 minutes on ice. FACs buffer consisted of HBSS (Sigma) with 2% bovine calf serum (Sigma), 0.1% HEPES and 0.1% sodium azide (Sigma). Next, the cells were stained for 1 hour on ice for the following cell-surface markers: anti-mouse CD8a PE/Cy7 (clone 53-6.7, Biolegend), CD137 eFluor450 (clone 17B5, eBioscience, San Diego, CA, USA), PD-1 FITC (clone RMP1-30, eBioscience), CD3 APC-Cy7 (clone 145-2C11, BD Biosciences), CD11b PE-Texas Red (clone M1/70.15, Invitrogen), F4/80 PE-Cy7 (BM8, eBioscience), Ly6C PerCP-Cy5.5 (HK1.4, eBioscience) or Ly6G V450 (1A8, BD Biosciences). If the cells were not stained for interferon-γ (IFN-γ), then they were washed twice with FACs buffer and resuspended in FACS buffer, and flow cytometry was performed with the Gallios flow cytometer (Beckman Coulter).
If the cells were stained for IFN-γ, then the cells were washed twice with PBS, resuspended and incubated in Fixation/Permeabilization buffer (Life Technologies) at 4 °C for 30 minutes. The cells were washed twice with Permeabilization buffer (Life Technologies), and incubated with IFN-γ APC (clone XMG1.2, eBioscience) for 30 minutes on ice. The cells were washed twice with Permeabilization buffer and resuspended in FACs buffer, and flow cytometry was performed with the Gallios flow cytometer.
Statistical analysis
Survival analysis was performed using the log-rank test. For comparison of cell percentage, cell number and cytokine expression between groups, the mean values were analyzed using unpaired one-way ANOVA with Tukey P value adjustment for multiple comparisons. Error bars on graphs represent standard deviation. All tests were two-tailed, and P<0.05 was considered statistically significant.
Results
Combinational immunotherapy improves survival when administered after gemcitabine
Gemcitabine is a part of the standard regimen for the treatment of all stages of PDA, from localized to metastatic cancer, and as such, most patients have been exposed to this chemotherapy agent prior to entering clinical trials investigating immunotherapy. In addition, when chemotherapy is administered prior to immunotherapy, chemotherapy-induced tumor cell apoptosis supplies an antigen-source for immunotherapy (5,14). Finally, some report that chemotherapy-induced PD-L1 expression may be transient, with Peng et al. reporting that patients with advanced ovarian cancer treated with paclitaxel and carboplatin had increased PD-L1+ tumor cells in the ascites, but that this decreased 11 days after chemotherapy exposure (2). Thus, to take advantage of the chemotherapy-primed TME, we sequenced the regimen such that gemcitabine was administered first, followed by the combination immunotherapy 4 days later (Figure 1A). We employed the previously described mouse model of hepatic metastatic PDA, where KPC tumor cells were injected into a clipped hemi-spleen on day 0 (27). Treatment with gemcitabine started 4 days after tumor inoculation, and 2 doses were given 3 days apart (i.e., on days 4 and 7). On day 11, immunotherapy with GVAX, αPD-1 and/or αCSF-1R was initiated, and GVAX was continued weekly and αPD-1 and/or αCSF-1R were continued twice a week for a total of 3 weeks. Adding combinational immunotherapy to gemcitabine improved survival compared to gemcitabine alone, whether it be GVAX + αPD-1, αPD-1 + αCSF-1R or GVAX + αPD-1 + αCSF-1R with the gemcitabine (Figure 1B). There was no statistically significant difference in the survival between any of the groups that received both chemotherapy and immunotherapy (Figure 1B). The survival of the untreated group and the immunotherapy alone groups from the same experiments have been previously reported (25).
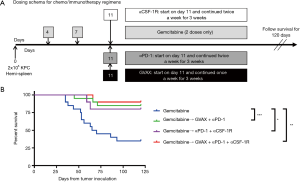
Administering combinational immunotherapy after gemcitabine therapy increases infiltration of CD8+ effector T-cells
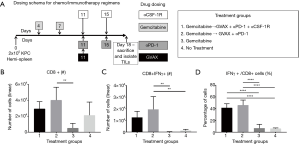
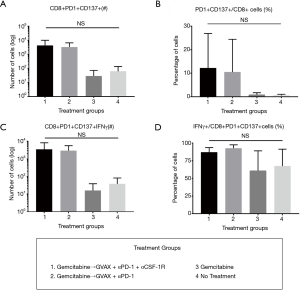
To determine how the immunotherapy conferred a survival benefit when combined with gemcitabine, we analyzed the metastatic PDA-infiltrating T-cells with flow cytometry after the mice received 2 weeks of therapy (Figure 2A). The mice that received only gemcitabine yielded a statistically significant lower number of tumor-infiltrating CD8+ T-cells compared to the gemcitabine + GVAX + αPD-1 treatment group, and trended towards a lower number of CD8+ T-cells compared to gemcitabine + GVAX + αPD-1 + αCSF-1R treatment and even the group that did not receive treatment (Figure 2B). We also found that gemcitabine/immunotherapy groups had a higher number and percentage of CD8+ T-cells that expressed IFN-γ compared to gemcitabine-only and no treatment groups, suggesting that gemcitabine/immunotherapy combination therapy increased infiltration of effector CD8+ T-cells into the tumors (Figure 2C,D). We previously found that the combination of GVAX + αPD-1 + αCSF-1R increased the percentage and number of CD8+ T-cells that expressed both PD-1 and CD137, and we demonstrated that these cells were effector T-cells (25). Accordingly, we analyzed if these markers, PD-1 and CD137, were increased amongst the tumor-infiltrating CD8+ T-cells. In this study, the gemcitabine/immunotherapy groups trended towards an increased number and percentage of double-positive CD8+PD1+CD137+ cells (Figure 3A,B) and a higher number of CD8+PD1+CD137+IFN-γ+ cells (Figure 3C) compared to the gemcitabine-only and no treatment groups, but they were not statistically significant. As observed in our previous study, these double-positive CD8+ T-cells expressed high levels of IFN-γ (Figure 3D) (25). It is interesting that the gemcitabine/immunotherapy group that did not contain αCSF-1R (i.e., gemcitabine + GVAX + αPD-1) also increased the infiltration of double-positive cells, because we had previously found that GVAX + αPD-1 therapy alone did not increase the presence of these double-positive cells (25).
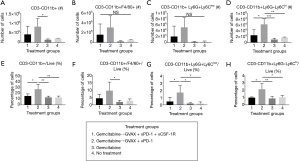
CSF-1R inhibition in combination with gemcitabine, GVAX and PD-1 inhibition decreases CD11b+ myeloid cells within the TME
We then investigated the effect of our treatment regimen on the myeloid cell population within the liver-metastatic PDA tumors. GVAX has previously been shown to increase myeloid cell infiltration into PDA tumors, which we again observed in this study (Figure 4) (21,25). The treatment groups that contained GVAX had higher infiltration of myeloid cells (CD3-CD11b+) and myeloid cell subsets: TAMs (CD3-CD11b+F4/80+), G-MDSCs (CD3-CD11b+Ly6G+Ly6Clow) and M-MDSCs (CD3-CD11b+Ly6G-Ly6Chi) (Figure 4). When CSF-1R inhibition was added to the gemcitabine + GVAX + αPD-1 regimen, αCSF-1R was able to statistically significantly decrease the percentage of myeloid (CD3-CD11b+) cells, and specifically the MDSCs, amongst the tumor-infiltrating immune cells (Figure 4E,G,H). There was also a trend towards a decreased number and percentage of infiltrating TAMs with the addition of αCSF-1R to the gemcitabine + GVAX + αPD-1 regimen (Figure 4B,F).
Discussion
The administration of immunotherapy after chemotherapy improved survival in this study. It is reassuring that sequencing immunotherapy after chemotherapy does not abrogate the survival benefit provided by chemotherapy given that, currently, most cancer patients have been exposed to chemotherapy prior to initiation of immunotherapy. However, the duration between the two types of therapies very likely plays a role in the ultimate therapeutic effect, and this was not investigated in this study (2).
While gemcitabine caused a trend towards decreased immune cell infiltration, immunotherapy was able to induce immune cell infiltration into the tumor even when administered after gemcitabine. Adding GVAX and αPD-1, with or without αCSF-1R, to gemcitabine led to almost a 7-fold increase in the percentage of tumor-infiltrating IFN-γ-expressing CD8+ T-cells (Figure 2D) and 12-fold increase in the percentage of PD1+41BB+ CD8+ T-cells (Figure 3D). The addition of αCSF-1R therapy to gemcitabine + GVAX + αPD-1 reversed the infiltration of myeloid cells caused by GVAX back to gemcitabine-alone and no treatment levels (Figure 4). The high level of activated effector CD8+ T-cells infiltrating the tumor in chemo-immunotherapy treatment groups compared to gemcitabine-only and no treatment groups, suggests that the addition of chemotherapy did not have detrimental effects on cytotoxic T-cell function. However, further comparisons with immunotherapy-only groups would be necessary to confirm this.
Perhaps a different dose or dosing schedule with αCSF-1R would have further decreased the infiltration of the tumor-promoting myeloid cells, particularly the TAMs which only trended down. Conceivably then the addition of αCSF-1R to gemcitabine + GVAX + αPD-1 would have led to a survival benefit compared to regimens without it. In addition, as has been demonstrated in prior studies, Figure 4A,E show that gemcitabine alone decreased CD11b+ cell infiltration into the tumor, and thus it is likely that gemcitabine was functioning similarly to αCSF-1R and depleting the tumor-promoting myeloid cells (15). The similarity in effect of gemcitabine and αCSF-1R may explain the lack of survival difference between gemcitabine + GVAX + αPD-1 and gemcitabine + GVAX + αPD-1 + αCSF-1R.
There was also no difference in survival between the gemcitabine + αPD-1+ αCSF-1R treatment group and the two gemcitabine/immunotherapy treatment groups that contained GVAX (Figure 1). We speculate that the tumor cell death caused by gemcitabine released the tumor-associated antigens and thus gemcitabine functioned similarly to a cancer vaccine, and primed the T-cells (14,16). After gemcitabine primed the TME, αPD-1 removed the immunosuppressive brakes, and if αCSF-1R was present, it prevented further infiltration of tumor-suppressive myeloid cells, with both actions leading to the activation of primed T-cells. Further analysis of the tumor-associated immune cells associated with this regimen would need to be performed to support this hypothesis.
The results of this study suggest that the redundancies in mechanisms of action between different agents within a combination treatment regimen (e.g., gemcitabine and GVAX priming the TME, and gemcitabine and αCSF-1R decreasing myeloid infiltration into tumor) may not lead to the hoped for improvement in anti-tumor efficacy and survival. In this era of ever-increasing complexity of cancer therapy regimens, this study highlights the importance of creating combination regimens that are based on scientific rationale, where the different agents in the treatment have demonstrable synergy and complementary anti-tumor effects.
Conclusions
This study demonstrates that the addition of immunotherapy can improve upon the survival conferred by a chemotherapy regimen in a murine model of PDA. However, a study with an orthotopically implanted tumor model has limitations. Our study suggests that combining chemotherapy, the current standard of care for PDA, with αPD-1, αCSF-1R and vaccine therapy can lead to a strong anti-tumor immune response; however, their synergistic effect remains to be further validated through human clinical trials.
Acknowledgments
Funding: This work was supported in part by the ASCO Young Investigator Award (MTS), NIH T32 CA 9071–36 (MTS), a BMS II-ON grant (LZ), NIH grant R01 CA169702 (LZ), NIH grant R01 CA197296 (LZ), the Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (LZ), National Cancer Institute Specialized Programs of Research Excellence in Gastrointestinal Cancers grant P50 CA062924 (LZ) and Sidney Kimmel Comprehensive Cancer Center grant P30 CA006973 (LZ).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2019.11.01). LZ serves as the Editors-in-Chief of Annals of Pancreatic Cancer. LZ receives grant support from Bristol-Meyer Squibb, Merck, iTeos, Amgen, NovaRock, Inxmed, and Halozyme, and received the royalty for licensing GVAX to Aduro Biotech. LZ is a paid consultant/Advisory Board Member at Biosion, Alphamab, NovaRock, Akrevia, Sound Biologics, Fusun Biopharmaceutical, Foundation Medicine, Datarevive, and Mingruzhiyao. LZ holds shares at Alphamab and Mingruzhiyao. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animals are cared for in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
Disclaimer: This article was prepared while May Tun Saung was employed at Johns Hopkins University. The opinions expressed in this article are the author’s own and do not reflect the view of the Food and Drug Administration, the Department of Health and Human Services, or the United States government.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35. [Crossref] [PubMed]
- Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κBto foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res 2015;75:5034-45. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- O'Hara MH, O'Reilly EM, Rosemarie M, et al. A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Available online: https://cancerres.aacrjournals.org/content/79/13_Supplement/CT004
- Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005;202:1691-701. [Crossref] [PubMed]
- Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005;123:321-34. [Crossref] [PubMed]
- Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007;13:54-61. [Crossref] [PubMed]
- Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050-9. [Crossref] [PubMed]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998;392:86-9. [Crossref] [PubMed]
- Friesen C, Herr I, Krammer PH, et al. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med 1996;2:574-7. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 2003;170:4905-13. [Crossref] [PubMed]
- Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005;11:6713-21. [Crossref] [PubMed]
- Liu WM, Fowler DW, Smith P, et al. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer 2010;102:115-23. [Crossref] [PubMed]
- Plate JM, Plate AE, Shott S, et al. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother 2005;54:915-25. [Crossref] [PubMed]
- Bauer C, Bauernfeind F, Sterzik A, et al. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 2007;56:1275-82. [Crossref] [PubMed]
- Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014;2:616-31. [Crossref] [PubMed]
- Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T cell infiltration into pancreatic tumors. J Immunother 2015;38:1-11. [Crossref] [PubMed]
- Tsujikawa T, Kumar S, Borkar RN, et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Reports 2017;19:203-17. [Crossref] [PubMed]
- Dai XM, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002;99:111-20. [Crossref] [PubMed]
- Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128-41. [Crossref] [PubMed]
- Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014;74:5057-69. [Crossref] [PubMed]
- Saung MT, Muth S, Ding D, et al. Targeting myeloid-inflamed tumor with anti-CSF-1R antibody expands CD137+ effector T-cells in the murine model of pancreatic cancer. J Immunother Cancer 2018;6:118. [Crossref] [PubMed]
- Foley K, Rucki AA, Xiao Q, et al. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Sci Signal 2015;8:ra77. [Crossref] [PubMed]
- Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469-83. [Crossref] [PubMed]
- Soares KC, Foley K, Olino K, et al. A preclinical murine model of hepatic metastases. J Vis Exp 2014;51677. [PubMed]
Cite this article as: Saung MT, Zheng L. Adding combination immunotherapy consisting of cancer vaccine, anti-PD-1 and anti-CSF1R antibodies to gemcitabine improves anti-tumor efficacy in murine model of pancreatic ductal adenocarcinoma. Ann Pancreat Cancer 2019;2:21.


