
Analyzing outcomes of neoadjuvant and adjuvant treatment for borderline-resectable pancreatic adenocarcinoma in the perioperative period at an academic institution
Introduction
Pancreatic adenocarcinoma (PDAC) carries a high mortality rate, ranking number four out of all cancer-related deaths, second for gastrointestinal malignancies in the United States (1) and seventh worldwide (2). However, this is expected to change with cancer-related death from PDAC reaching 2nd place by 2030 (3). The only potentially curative modality is complete resection, followed by adjuvant therapy; unfortunately, only approximately 15–20% of patients are surgical candidates at presentation due to delayed development of symptoms and consequent late diagnosis (4). In addition, among patients who undergo surgical resection, only approximately 20% are alive at 5 years (5). Most patients have symptomatic disease at diagnosis due to locally advanced pancreatic cancer. Only a selected number of patients with good performance status and “borderline-resectable” disease can undergo perioperative therapy and surgical resection. Patients with unresectable and/or metastatic disease are offered palliative chemotherapy.
Determination of surgical resectability in localized disease is a spectrum that ranges from resectable, borderline-resectable, to unresectable depending on the extent of vascular invasion, infiltration of adjacent structures, and involvement of distal lymph nodes based on a criterion established by the National Comprehensive Cancer Network (NCCN) (6). Tumors that encase the celiac artery and superior mesenteric artery (>180 degrees), with unreconstructable portal vein and superior mesenteric vein, and involvement of non-regional lymph node metastasis are considered unresectable; conversely, tumors that completely spare the above-mentioned vasculatures are considered resectable (6). Borderline-resectability is generally considered for tumors that abut the arterial vasculature at <180 degrees and/or about the portal or inferior vena cava vein (6). In clinical practice this translates to defining tumors of large dimension T3 (size >4 cm) or any T4 (contact with vasculature regardless of size) for stages IIA, IIB and III (4) as borderline-resectable. The treatment for resectable pancreatic cancer continues to be upfront surgery; unfortunately, even for patients who achieve complete resection and are treated with adjuvant therapy, the 5-year recurrence rate is ~80% and the 5-year survival rate is between 5% to 25% (7).
Based on mature data, the American Society of Clinical Oncology (ASCO) recommends adjuvant chemotherapy for 6 months after surgical resection for patients who did not receive perioperative therapy (8). Available adjuvant treatment options are gemcitabine monotherapy [2007 Conko-1 (9)], gemcitabine-capecitabine combination [2017 ESPAC-4 (10)], or modified FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, and short-term infusional fluorouracil, [2018 PRODIGE-24 (11)], which provide 22.8, 28.0, and 54.4 months median overall survival (mOS), respectively. The selection of each regimen is based on provider preference and patient’s performance status and age.
The role of neoadjuvant therapy for patients with resectable disease is undefined, and its use continues to evolve. The European Society of Medical Oncology (ESMO), NCCN, and ASCO recommend against neoadjuvant therapy for those with resectable pancreatic cancer outside of a clinical trial (6,8,12). However, in clinical practice, a majority of patients are considered to have “borderline-resectable” locally advanced disease given suspicious lesions on imaging, blood vessel involvement, and elevated CA 19-9 levels. The SWOG 1505 trial (NCT02562716), which is studying neoadjuvant chemotherapy for patients with resectable pancreatic cancer, reported a 29% rate of screen fail; of those cases, 36% had venous involvement ≥180°, 52% had extensive arterial involvement, and 67% had occult distant disease discovered upon central radiology review (13). Due to controversial and inadequate data highlighting a benefit for neoadjuvant therapy for borderline-resectable disease, current guidelines recommend enrolling patients to clinical trials. If clinical trials are unavailable or patients are ineligible to participate, the guidelines recommend considering chemotherapy (gemcitabine-based or mFOLFIRINOX) followed by chemo-radiation when applicable (chemo-sensitizers, including 5-fluorouracil, gemcitabine, capecitabine), and assessing response prior to surgery [Evidence Category 2A, 6,8,12]. There are insufficient data to offer recommendations for perioperative therapy (neo and adjuvant treatments combined) to patients with “borderline-resectable” pancreatic cancer due to the lack of randomized controlled clinical trials showing benefit. The best regimen and modality remain undefined.
Our primary objective was to determine the mOS of different treatment modalities for patients with “borderline-resectable” pancreatic cancer at a large academic center, focusing on surgery (S) alone, neoadjuvant alone [neoadjuvant followed by surgery (NeoS)], adjuvant alone [surgery followed by adjuvant (SA)], or neoadjuvant plus adjuvant [perioperative therapies (NeoSA)] groups. Our secondary objectives were to analyze PFS and DFS of each group, and to further analyze resection margins and outcomes of radiation therapy.
Methods
A retrospective chart review using electronic medical records (EMR) of patients with “borderline-resectable” pancreatic cancer who underwent conventional (“Whipple”) or modified pancreaticoduodenectomy surgery between November 2011 and December 2016 at the University of Arizona Cancer Center was collected utilizing the cancer registry. The study was approved by the institutional review board of the University of Arizona (Protocol Number: 1804508070). Inclusion criteria were: (I) histologically proven pancreatic ductal adenocarcinoma (PDAC); (II) available radiological evaluation by triple-phase contrast-enhanced computed tomography, magnetic resonance imaging, or endoscopic ultrasound of a “borderline-resectable” tumor (Table 1) (clinical stage IIA, IIB, or III); (III) Eastern Cooperative Oncology Group (ECOG) performance status score of ≤2 and appropriate comorbidity profile for surgery and systemic therapy; and (IV) available perioperative EMR documentation. Exclusion criteria were: (I) patient with another malignancy not related to PDAC; (II) clinically resectable disease (Stage I), and unresectable locally advanced or metastatic pancreatic cancer (Stage IV); (III) surgical intervention aborted; (IV) other documented infectious or autoimmune active disease; and (V) incomplete medical documentation (pathology, scans, tumor board, or any other documents).
Table 1
| Category by TNM (a) | Clinical stage | Resectability criteria (b) | Guideline consensus recommendations (c, d, e) |
|---|---|---|---|
| T1-3, N0, M0 | 0-IIA | Resectable | Upfront surgery followed by adjuvant therapy |
| T1-3, N1-2, M0 | IIB-III | Borderline-resectable | Consider neoadjuvant therapy prior surgery |
| T4^, N any, M0 | III | Locally advanced unresectable^ | Consider induction systemic therapy followed by chemoradiation therapy consolidation |
a, Tumor, Node, Metastasis (TNM) system of the combined American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) eighth edition, 201704; b, Pancreatic Adenocarcinoma, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. Journal National Comprehensive Cancer Network. 201906; c, Guidelines of the American Society of Clinical Oncology08 (ASCO), the National Comprehensive Cancer Network06 (NCCN) and the European Society for Medical Oncology12 (ESMO); d, participation in clinical trials is encouraged; e, multidisciplinary and comprehensive cancer care discussion is advised; ^, T4 Tumor involves >180º celiac axis, superior mesenteric artery, and/or common hepatic artery, and/or unreconstructible superior mesenteric vein/portal vein due to tumor involvement or occlusion, regardless of size.
Patient demographics including age, gender, race, ethnicity, and comorbidities were collected. A preoperative level of the serum tumor marker carbohydrate antigen 19-9 (CA 19-9; reference range, 0–37 U/mL) was included. Clinical-radiological stage was recorded by tumor size (cm, cT1-4), regional lymph node status (cN0-2), and final TNM 8th Edition Staging System (cIIA-III), and was reviewed and assessed by a hepatobiliary radiologist. Pathologic staging was also recorded by tumor location, histologic and differentiation grade (well, moderate, and poor), lymph node involvement (pN0-2), and resection margins (R0, R1, and R2). These were obtained from the surgical pathology report. The resection margins were defined as R0 for complete resection with all margins histologically uninvolved, R1 for incomplete resection with microscopic surgical resection margin involvement, and R2 for incomplete with a gross residual tumor that was not resected. Therapeutic treatment regimens including systemic chemotherapy, radiation therapy, or participation in a clinical trial were recorded.
Patients were then divided by treatment modality: Neoadjuvant + surgery + adjuvant (NeoSA), surgery + adjuvant (SA), neoadjuvant + surgery (NeoS), and surgery alone. OS was defined as the time from diagnosis until death from any cause, with patients alive or lost to follow-up at the cut-off date considered censored. PFS was defined as the time from biopsy-proven diagnosis until objective tumor progression (First-line treatment) or death, whichever occurs first. DFS was defined as the time from surgical resection until disease recurrence (First-line treatment) or death from any cause. Patients (n=3) with unknown progression status or unknown disease-free status were excluded from the PFS and DFS analyses.
Continuous data were summarized using means, standard deviations, medians and interquartile ranges. Categorical data were summarized using frequency tables. Continuous normally distributed data were compared using ANOVA methods. Continuous non-normally distributed data were compared using the non-parametric Kruskal-Wallis Rank Sum test. Categorical data were compared using χ2 or Fisher’s exact test. OS, PFS, DFS were summarized graphically using Kaplan-Meier methods while comparisons across treatment regimens used Log Rank tests. Multivariate Cox regression analysis was performed to assess the association of demographic and tumor traits with OS, PFS and DFS. Adjustment variables were radiation (yes vs. no), resection margins (R0 vs. R1 or R2), and tumor location (head vs. body or tail). Two cases with “overlapping” tumor location were removed from the adjusted analyses. Additional sensitivity analyses adjusted for demographics variables, CA 19-9 tumor marker and ECOG status; the overall results were similar so are not presented. Logistic regression was used to assess differences in odds of death by treatment regimen. Statistical analyses were performed using Stata15. (Stata Corp, College Station, TX, USA). Statistical significance was set at P value less than 0.05.
Results
Our search of the University of Arizona Cancer Center tumor registry identified a total of 73 patients who fulfilled eligibility criteria for borderline-resectable pancreatic cancer. Of those patients, perioperative treatment modalities were divided into NeoSA (n=9), SA (n=40), NeoS (n=9) and S (n=15). Our analysis included patients with a mean age of 65.7 years old at diagnosis (range, 41–92), with 56.2% male, 86.3% white, and 80.8% non-Hispanics, 83.6% had ECOG of 0-1, 64.4% had biliary stent placement, and preoperative CA 19-9 mean was 547.5 U/mL (SD 1,116.7, Table 2). Clinical radiology stages were II (87.6%) and III (12.3%). Pathology report highlighted: 83.6% at head of pancreas, 65.7% with moderate histological differentiation, 63.0% N1 lymph node positive, and positive resection margins in 50.7% of patients (R1 & 2, Table 3).
Table 2
| Characteristic | Surgery alone (N=15) | Neoadjuvant therapy (N=9) | Adjuvant therapy (N=40) | Neoadjuvant & adjuvant therapy (N=9) | Total (N=73) | P value |
|---|---|---|---|---|---|---|
| Age at diagnosis-years, mean (SD) | 70.6 (8.6) | 61.7 (12.9) | 65.4 (10.3) | 63.1(7.1) | 0.1341 | |
| >65 years old | 11 (73.3%) | 5 (55.6%) | 18 (45.0%) | 4 (44.4%) | 38 (52.1%) | 0.2802 |
| Gender | 0.3902 | |||||
| Female | 9 (60.0%) | 5 (55.6%) | 15 (37.5%) | 3 (33.3%) | 32 (43.8%) | |
| Male | 6 (40.0%) | 4 (44.4%) | 25 (62.5%) | 6 (66.7%) | 41 (56.2%) | |
| Race | 0.1512 | |||||
| White | 12 (80.0%) | 7 (77.8%) | 37 (92.5%) | 7 (77.8%) | 63 (86.3%) | |
| African-American | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) | 1 (11.1%) | 2 (2.7%) | |
| American Indian | 1 (6.7%) | 1 (11.1%) | 2 (5.0%) | 0 (0.0%) | 4 (5.5%) | |
| Asian | 1 (6.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) | |
| Unknown | 1 (6.7%) | 1 (11.1%) | 0 (0.0%) | 1 (11.1%) | 3 (4.1%) | |
| Ethnicity | 0.5882 | |||||
| Non-Hispanic | 12 (80.0%) | 6 (66.7%) | 34 (85.0%) | 7 (77.8%) | 59 (80.8%) | |
| Hispanic | 3 (20.0%) | 2 (22.2%) | 5 (12.5%) | 2 (22.2%) | 12 (16.4%) | |
| Unknown | 0 (0.0%) | 1 (11.1%) | 1 (2.5%) | 0 (0.0%) | 2 (2.7%) | |
| Performance status ECOG score | 0.0332 | |||||
| 0 | 0 (0.0%) | 2 (22.2%) | 11 (27.5%) | 4 (44.4%) | 17 (23.3%) | |
| 1 | 10 (66.7%) | 7 (77.8%) | 24 (60.0%) | 3 (33.3%) | 44 (60.3%) | |
| 2 | 5 (33.3%) | 0 (0.0%) | 5 (12.5%) | 2 (22.2%) | 12 (16.4%) | |
| Preoperative CA 19-9 mean U/mL (SD) | 217.9 (287.9) | 1,285.6 (1,732.4) | 282.0 (487.8) | 1,444.7 (2,117.7) | 547.5 (1,116.7) | 0.0053 |
| Biliary stent placement | 10 (66.7%) | 5 (55.6%) | 26 (65%) | 6 (66.7%) | 47 (64.3) | 0.8542 |
1, ANOVA; 2, Fisher’s Exact Test; 3, Kruskal-Wallis Rank Sum Test. ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen 19-9.
Table 3
| Characteristic | Surgery alone | Neoadjuvant therapy | Adjuvant therapy | Neoadjuvant & adjuvant therapy | Total | P value |
|---|---|---|---|---|---|---|
| Stage AJCC/TNM | 0.2092 | |||||
| IIA | 4 (26.7%) | 2 (22.2%) | 11 (27.5%) | 2 (22.2%) | 19 (26.0%) | |
| IIB | 10 (66.7%) | 3 (33.3%) | 25 (62.5%) | 7 (77.8%) | 45 (61.6%) | |
| III | 1 (6.7%) | 4 (44.4%) | 4 (10.0%) | 0 (0.0%) | 9 (12.3%) | |
| Location | 0.6592 | |||||
| Body | 1 (6.7%) | 2 (22.2%) | 2 (5.0%) | 1 (11.1%) | 6 (8.2%) | |
| Head | 12 (80.0%) | 7 (77.8%) | 34 (85.0%) | 8 (88.9%) | 61 (83.6%) | |
| Overlapping pancreas | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) | 0 (0.0%) | 2 (2.7%) | |
| Tail | 2 (13.3%) | 0 (0.0%) | 2 (5.0%) | 0 (0.0%) | 4 (5.5%) | |
| Histological differentiation | 0.2742 | |||||
| Well | 1 (6.7%) | 2 (22.2%) | 4 (10.0%) | 1 (11.1%) | 8 (10.9%) | |
| Moderate | 8 (53.3%) | 7 (77.8%) | 27 (67.5%) | 6 (66.7%) | 48 (65.7%) | |
| Poor | 6 (40.0%) | 0 (0.0%) | 10 (25%) | 1 (11.1%) | 17 (23.2%) | |
| Lymph node positive | 0.0972 | |||||
| N0 | 7 (46.6%) | 4 (44.4%) | 11 (27.5%) | 2 (22.2%) | 24 (32.8%) | |
| N1 | 8 (53.3%) | 5 (55.6%) | 27 (67.5%) | 6 (66.7%) | 46 (63.0%) | |
| N2 | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) | 1 (11.1%) | 3 (4.1%) | |
| Resection margins | 0.0512 | |||||
| R0 | 4 (26.7%) | 7 (77.8%) | 20 (50.0%) | 5 (55.6%) | 36 (49.3%) | |
| R1 | 3 (20.0%) | 0 (0.0%) | 12 (30.0%) | 4 (44.4%) | 19 (26.0%) | |
| R2 | 8 (53.3%) | 2 (22.2%) | 8 (20.0%) | 0 (0.0%) | 18 (24.6%) |
1, ANOVA; 2, Fisher’s Exact Test; 3, Kruskal-Wallis Rank Sum Test. AJCC/TNM 8th Ed. = American Joint Committee on Cancer/Tumor, Node, Metastasis Eight Edition; N, nodes, R, resection.
The most common chemotherapeutic regimens were mFOLFIRINOX (77.7%) in the neoadjuvant setting, gemcitabine-based (95.9%) in the adjuvant setting, and gemcitabine-nab-paclitaxel in the first-line (38.8%) and second-line treatment (37.5%). Chemo-radiotherapy (61.1%) and clinical trials (22.2%) were more frequently used in the neoadjuvant setting (Table 4). The median time in weeks from diagnosis to surgery was 5.3, from diagnosis to neoadjuvant was 4.7, and from surgery to adjuvant was 8.9 (Table 4). The median follow-up time (date of diagnosis to date of last contact) for all patients was 17.7 months.
Table 4
| Treatment modality | Therapeutic regimen | n (%) |
|---|---|---|
| Neoadjuvant | mFOLFIRINOX | 14 |
| 18 participants received 33 therapeutic regimens* | Gemcitabine-based | 4 |
| Chemotherapy followed by chemo-radiation therapy | 11 | |
| Clinical trial | 4 | |
| Adjuvant | Gemcitabine-based | 47 |
| 49 participants received 70 therapeutic regimens* | Chemotherapy followed by chemo-radiation therapy | 14 |
| Clinical trial | 7 | |
| mFOLFIRINOX | 2 | |
| Perioperative radiation protocol (gray/fractions); N25 (%) | 50.4 Gy/28 Fx | 18 (72.0) |
| 33 Gy/5–10 Fx | 4 (16.0) | |
| Unknown | 3 (12.0) | |
| First-line treatment; N36 (%) | Gemcitabine-nab-paclitaxel | 14 (38.8) |
| mFOLFIRINOX | 8 (22.2) | |
| CAPOX | 7 (19.4) | |
| Chemo-radiation therapy followed by chemotherapy | 3 (8.3) | |
| FOLFOX | 2 (5.5) | |
| Nanoliposomal irinotecan-based | 1 (2.7) | |
| FOLFIRI | 1 (2.7) | |
| Second-line treatment N16 (%) | Gemcitabine-nab-paclitaxel | 6 (37.5) |
| CAPOX | 4 (25.0) | |
| Nanoliposomal irinotecan-based | 3 (18.7) | |
| mFOLFIRINOX | 2(12.5) | |
| Chemo-radiation therapy followed by chemotherapy | 1 (6.2) | |
| Time to surgery from diagnosis (weeks) | 5.3 | |
| Time to neoadjuvant from diagnos is (weeks) | 4.7 | |
| Time to adjuvant from surgery (weeks) | 8.9 |
*, patients may appear in multiple treatment regimens on the neoadjuvant and adjuvant treatment modalities. mFOLFIRINOX, modified FOLinic acid-Fluorouracil-IRINotecan-OXaliplatin; CAPOX, CAPecitabine-Oxaliplatin; FOLFOX, FOLinic acid-Fluororuracil-OXaliplatin; FOLFIRI, FOLinic acid, Fluorouracil, IRInotecan.
The unadjusted median OS times were: 11.3 months for surgery only, 17.7 months for NeoS, 32.4 months for NeoSA, and 45.2 months for SA (P=0.0002) (Figure 1A). After adjustment for clinically relevant covariates (radiation, resection margins, tumor location), the SA group had a significantly improved hazard ratio compared with the S group (HR =0.22; 95% CI, 0.09–0.51; P<0.001). SA patients had 2.1 times greater odds to be alive at 24 months than surgery alone (P=0.015). Neither the NeoS nor NeoSA treated patients had a statistically significant increase in survival when compared to surgery alone (P=0.71 and P=0.53, respectively). Those whose disease was located in the head of the pancreas had a significantly improved hazards ratio (HR =0.27; 95% CI, 0.11–0.64; P=0.003). Those who received radiation had a non-significantly improved hazards ratio (HR =0.40; 95% CI, 0.16–1.04; P=0.06), as did those with negative margins (HR =0.48; 95% CI, 0.22–1.03; P=0.06).
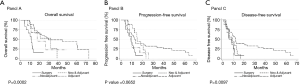
The unadjusted median PFS was 9.9 months for surgery only, 8.8 months for NeoS, 12.7 months for SA, and 15.0 months for NeoSA. There was no statistically significant difference in unadjusted PFS by treatment regimen (P=0.07) (Figure 1B). Negative margins led to a significant improvement in PFS (HR =0.30; 95% CI, 0.16–0.57 P<0.001), as did tumor location in the head of the pancreas (HR =0.40; 95% CI, 0.17–0.94; P=0.035). Those who received radiation had a non-significantly improved hazards ratio (HR =0.49; 95% CI, 0.24–1.01; P=0.054) for adjusted PFS.
The unadjusted median DFS was 5.9 months for surgery only, 8.8 months for NeoS, 9.1 months for NeoSA and 12.0 months for SA. There was a statistically significant difference in median unadjusted DFS by treatment regimen (P=0.0097) (Figure 1C), however, there were no statistically significant differences after adjustment (P=0.092, P=0.100, P=0.399 for SA, NeoS, and NeoSA, respectively). Negative margins led to a significant improvement in DFS (HR =0.30; 95% CI, 0.16–0.57; P<0.001), as did tumor location in the head of the pancreas (HR =0.30; 95% CI, 0.13–0.67; P=0.004). Those who received radiation had a non-significantly improved hazards ratio (HR =0.54; 95% CI, 0.26–1.10; P=0.090) for adjusted DFS.
Discussion
Currently, oncologists and thought leaders have no consensus recommendations for the best treatment course for patients with “borderline-resectable” pancreatic cancer. In our retrospective review of patients with “borderline-resectable PDAC”, post-operative treatment consisting of “SA therapy”, was the only approach to demonstrate an OS benefit. The mOS of our adjuvant therapy SA group (45 months) was numerically superior to those reported on prior phase III clinical trials [23 months, Conko-1 (9), 23 months ESPAC3 (14), 26 months JASPAC 01 (15), and 28 months ESPAC4 (10)], which is relatively similar as most of our patients (96%) received gemcitabine-based therapy per clinical practice guideline recommendations up to 2016 (7). Furthermore, the addition of standard radiation therapy (>70% of patients received 50.4 Gy/28 Fx protocol) to chemotherapy had an added non-statistically significant positive mOS trend with NeoS 11.2, NeoSA 9.9 and SA 29.8 months in patients without radiation versus mOS of 26.8, 32.4, and 61.3 months, respectively, in patients who received radiation (P=0.06) at our cancer center. In our experience, chemo-radiation benefit was more noticeable on those with high-risk features such as bulky head of the pancreas (T3) and positive resection margins (R1-2), indicating a potential role for radiation in this cohort of patients as indicated in ESMO, NCCN, and ASCO guidelines (6,8,12). Although CA 19-9 tumor marker and ECOG performance status were significantly different in the upfront surgery (including the adjuvant group) compared to the neoadjuvant groups, inclusion of these variables in the models did not alter the results. These were not detected as confounders nor did their inclusion independently influence the survival of patients in our analysis. This generally reflects common practice of offering surgery to patients with excellent performance status and low CA 19-9 levels (8).
In our study, the perioperative treatment (NeoSA) group had a positive mOS trend (32.4 months) compared to surgery alone (11.3 months) (P=0.025), however, there was no statistically significant effect after adjusting for all confounders (P=0.53). A meta-analysis of 21 studies (consisting of 1 phase I, 3 phase II, 6 prospective, and 11 retrospective) reported the mOS after neoadjuvant and adjuvant therapy in 881 patients with “borderline-resectable” PDAC as 19.2 months (95% CI, 11–32) compared to 12.8 months (95% CI, 11.6–16.3) for upfront surgery (16). In a retrospective study, the mOS of 63 “borderline-resectable” PDAC patients who received neoadjuvant treatment was similar to surgery alone, corroborated by our cohort with S 8.1 months and NeoS 11.2 months (17). PREOPANC-1, an ongoing randomized, controlled, multicenter phase II trial, is comparing patients with “borderline-resectable” PDAC receiving perioperative chemo-radiotherapy with gemcitabine, upfront surgery, adjuvant gemcitabine chemotherapy, or upfront surgery followed by 4 months of adjuvant chemotherapy (18). Preliminary data presented at the 2018 annual ASCO meeting showed the mOS was significantly better for the perioperative regimen (17.1 months) compared to adjuvant therapy alone (13.5 months), although adjuvant treatment [six cycles of gemcitabine alone] was considered substandard to current medicine-based adjuvant recommendations [six cycles of gemcitabine plus capecitabine on ESPAC4 (10), and twelve 2 weeks cycles of mFOLFIRINOX on PRODIGE-24 (11)], reflected in lower survival benefit when compared to historical controls (>20 months). In another comparable phase II trial, Yoo and colleagues presented at the 2019 annual ASCO meeting a trial of 44 patients who received 8 cycles of neoadjuvant mFOLFIRINOX, followed by 27 patients who have undergone surgery, of whom only 24 patients were able to complete 6 more cycles of adjuvant gemcitabine (19). Of those who completed perioperative therapy (NeoSA), mOS was 14.3 months, far below the published survival rates seen in adjuvant phase III trials (9-11). Prep-02/JSAP-05, a randomized, multicenter, phase II/III Japanese trial demonstrated a 36.7-month mOS benefit of neoadjuvant therapy with gemcitabine + oral S-1 [biosimilar to capecitabine not available in the US] followed by surgery and 6 months of adjuvant S-1. However, the study excluded “borderline-resectable” patients, and this regimen will potentially become a new standard for resectable PDAC in Japan (20). On a highly selected and small patient population, Murphy and colleagues’ single-arm phase II trial, gave preoperative 8 cycles of FOLFIRINOX followed by individualized chemo-radiotherapy (dose/fraction pending of treatment response) in borderline-resectable pancreatic cancer resulting in high rates of R0 resection (65%), prolonged median PFS (14.7 months) and median OS (not reached) (21). Prior mentioned results will further need corroboration from a phase III clinical trial, specifically to evaluate the added benefit of chemo-radiotherapy treatment strategy to systemic neoadjuvant plan noted by our retrospective review (mOS 26.8) although non-statistically powered.
Consistent with the ASCO, ESMO and NCCN guidelines, enrollment in a clinical trial is preferred and encouraged for all patients with “borderline-resectable” pancreatic cancer (6,8,12). Our university-based comprehensive cancer center only enrolled 16.5% of the potentially eligible “borderline-resectable” PDAC patients with pancreatic cancer between November 2011 and December 2016, parallel to the number reported in the “Pancreatic cancer clinical trials and accrual in the United States” of 15%, showing a missed opportunity to accelerate accrual and participation in clinical trials (22). If a clinical trial for a “borderline-resectable” PDAC patient is not available, the patient is not a trial candidate, or the patient is not interested in a clinical trial, then best clinical practice would suggest a short course of induction chemotherapy with mFOLFIRINOX for fit and young patients, or gemcitabine-nab-paclitaxel for those with borderline performance status (ECOG 2), comorbidities, or older patients (>65 years old). This can incorporate chemo-radiotherapy in patients with bulky disease as our study revealed a trend in improvement for mOS, albeit low-powered. It is recommended to proceed to surgery for those physically fit that achieved treatment response and we recommend adjuvant therapy based on the aforementioned phase III clinical trial results. Potential benefit could be attained from radiotherapy if surgical pathology shows positive resection margins, specifically if not administered in the neoadjuvant setting (23). However, to date, no specific chemo-radiation dose or schedule has been established. In the absence of prospective and randomized phase III trials demonstrating an OS benefit for perioperative treatment in patients with borderline PDAC, no overall recommendation can be established and management becomes individualized.
Our major study limitations were the low sample size, specifically in the neoadjuvant group as expected due to current clinical practice, and the retrospective nature of the study which may have missed other potential unknown confounding variables. In order to overcome these limitations, patients should be encouraged to participate in clinical trials (e.g., ALLIANCE trial A021501 NCT02839343, PANDAS-PRODIGE 44 NCT02676349, PRIMUS 002 ISRCTN34129115). Thus far, with current evidence-based medicine, patients with “borderline-resectable” pancreatic cancer should be offered upfront surgical resection followed by adjuvant chemotherapy or perioperative clinical trials.
Acknowledgments
We thank the University of Arizona Hematology and Medical Oncology Fellowship department for supporting trainees’ research.
Funding: 2P30 CA023074 supplement of the Cancer Center Support Grant from the NCI/NIH to the University of Arizona Cancer Center.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ apc.2020.02.01). HMB serves as an unpaid Section Editor of Annals of Pancreatic Cancer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the University of Arizona (Protocol Number: 1804508070). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 2017;24:2023-30.
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic Adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw 2019;17:202-10. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185-91.
- Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:2324-8. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56-68. Erratum in: Ann Oncol 2017;28:iv167-8. [Crossref] [PubMed]
- Sohal D, McDonough S, Ahmad SA, et al. SWOG S1505: Initial findings on eligibility and neoadjuvant chemotherapy experience with mfolfirinox versus gemcitabine/nab-paclitaxel for resectable pancreatic adenocarcinoma. J Clin Oncol 2019;37:414. [Crossref]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 2018;105:946-58. [Crossref] [PubMed]
- Kato H, Usui M, Isaji S, et al. Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: a multi-institutional survey by the Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013;20:601-10. [Crossref] [PubMed]
- Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36:LBA4002 [Crossref]
- Yoo C, Kim KP, Song KB, et al. Phase II trial of preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by post-operative gemcitabine (GEM) in patients (pts) with borderline-resectable pancreatic ductal adenocarcinomas (BR-PDAC). J Clin Oncol 2019;37:342. [Crossref]
- Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol 2019;37:189. [Crossref]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963-9. [Crossref] [PubMed]
- Hoos WA, James PM, Rahib L, et al. Pancreatic cancer clinical trials and accrual in the United States. J Clin Oncol 2013;31:3432-8. [Crossref] [PubMed]
- Recio-Boiles A, Babiker H. Pancreatic Adenocarcinoma: Approach to Adjuvant and Neoadjuvant Treatment. Hospital Physician Hematology/Oncology Board Review Manual. Volume 13, Part 2. March 2018.
Cite this article as: Recio-Boiles A, Vondrak J, Veeravelli S, Mancuso JJ, Saboda K, Roe DJ, Riaz IB, Scott AJ, Elquza E, McBride A, Babiker HM. Analyzing outcomes of neoadjuvant and adjuvant treatment for borderline-resectable pancreatic adenocarcinoma in the perioperative period at an academic institution. Ann Pancreat Cancer 2020;3:2.


