
Nonselective β-adrenergic blockade impacts pancreatic cancer tumor biology, decreases perineural invasion and improves patient survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease with high rates of disease-specific mortality. Surgical resection offers the only potential for cure, however, even in these selected populations, median survival remains poor with the overwhelming majority ultimately succumbing to disease (1-3). Aggressive tumor biology, a complex tumor microenvironment (TME) and broad resistance to existing chemotherapeutics are prominent drivers for these poor outcomes (1,4,5). The signaling mechanisms that mediate these processes remains poorly understood, but offers the potential for highly leveraged targets for therapeutic intervention.
The dense network of peripheral neurons that reside in the TME and interact closely with cancer cells is a component of particular interest in PDAC. Distinct neoplastic invasion into or around peritumoral nerves, or perineural invasion (PNI), is a common histologic feature of PDAC independently associated with inferior survival (6,7). Adrenergic signaling from peripheral sympathetic neurons has been implicated in the regulation of growth, progression and invasion of many cancer subtypes (8-10). Notable work investigating β-adrenergic signaling and its mechanistic relationship to the TME have been performed in the field of prostate cancer (11,12). Magnon et al. reported that the deletion of β2 and β3 adrenergic receptors prevented early phases of tumor development (11). While Zahalka et al. utilized mouse models of prostate cancer to show endothelial β2-adrenergic receptor signaling in the TME helped to activate angiogenesis exponentially fueling tumor growth (12). Thus, adrenergic neuron to cancer cell signaling may be involved in both the process of PNI as well as a general dictation of tumor invasiveness and biologic tumor behavior. Both epidemiologic and preclinical studies have suggested inhibition of this signaling via β-adrenergic blockade may reduce rates of tumor progression and improve survival outcomes for melanoma, breast, prostate and colon cancer (8,12-16). Similar associations of incidental β-blockade and improved survival have also been suggested in PDAC patients (17,18). β-blockers are widely available, safe and inexpensive, thus their use as a potential therapeutic adjunct for cancer treatment is immensely appealing.
In this study, we leverage a large, single institution’s experience of patients with resected PDAC to investigate the association between β-blockade and markers of tumor biology, including PNI and overall survival (OS). Furthermore, human-derived PDAC models are used to study this relationship in vitro and a proposed mechanism of tumor biology is derived.
Methods
Patient cohort
Clinicopathologic data for patients undergoing pancreatic resection at Johns Hopkins Hospital between 2000 and 2016 were collected in a prospectively maintained database and included in this study. Inclusion criteria included a confirmed diagnosis of PDAC upon final histopathologic analysis. Subsequently, cohorts were generated based on use of β-blockers in the perioperative period as determined by review of prescribed “home” medications at the time of resection. The specificity of prescribed β-blockade was identified: β1 selective (Atenolol, Bisoprolol, Metoprolol and Nebivolol) and Nonselective blockade (Propranolol, Carvedilol, Labetalol, Sotalol and Timolol). The primary outcomes of clinical interest were PNI and OS. Follow-up data through May 2018 were retrieved from the Institutional database. The date of death was obtained from medical records, local obituary, or the Social Security Death Index. OS was calculated from the date of surgery to the date of death from any cause, or censored at the date of last follow-up. This study was approved by the Institutional Review Board of Johns Hopkins Hospital (No. 00074221), conducted in accordance with the Declaration of Helsinki. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Demographics and clinicopathological characteristics
All patient data were collected from the prospectively maintained institutional database. Patient demographics included age, sex, race, history of hypertension (HTN), pain on presentation and β-blocker use. Neoadjuvant chemotherapy and/or radiation was noted as was the type of surgery: pancreaticoduodenectomy, distal or total pancreatectomy. Tumor pathology characteristics including tumor size, stage, grade of differentiation, PNI, lymphovascular invasion, presence of nodal disease and margin status were extracted from final pathology reports. Resections were considered “R0” when malignant cells were greater than 1 mm from the surgical margin.
Cell lines, growth and invasion assays
The human Panc10.05 pancreatic tumor cell line was obtained and established in accordance with IRB approved protocols. These cells were cultured in RPMI-1640 based medium supplemented with 10% FBS, 1% penicillin/streptomycin as previously described (19,20). Tumor cells were grown in 2D culture at 37 °C. Ten µM of norepinephrine and/or 1 µM of propranolol, atenolol and losartan potassium were added to culture media for growth and invasion assessments based on previous literature (21). In short, growth curves were analyzed by the addition of 1.5×104 cells resuspended equally in medium with or without supplemental norepinephrine and/or propranolol, atenolol and losartan potassium. At 0, 24, 48, 72, 96, and 120 hours, cells were counted and total number compared. Assays were run in biological quadruplicate and technical replicates were performed twice.
Invasion was estimated by scratch cell migration assay. Panc10.05 cells were grown in culture medium with or without supplemental norepinephrine and/or propranolol, atenolol and losartan potassium. Following 100% confluence, a scratch was performed, and closure percentage estimated at 0, 8, 24 and 32 hours was assessed to measure tumor cell migration. The area of the wound was obtained by the measurement of at least 6 random points per group. The area of closure was measured at each time point as:
Statistical analysis
Statistical analysis was performed using Stata/MP 12.1 (Stata Corp, College Station, TX). Categorical variables were expressed as percentages and compared using χ2 or Fisher exact test. Šidák correction was utilized for multiple comparisons. Continuous variables were presented as median and interquartile range (IQR) and compared using Kruskal-Wallis test. Kaplan-Meier curves were used to estimate median survival with the log-rank test utilized for subgroup comparison. A multivariate regression model was used with PNI as the outcome of interest. A Cox proportional-hazards regression model for OS was also used. For both models, a stepwise backward approach was used selecting all covariates with a P value <0.10 on univariate analysis used as the cutoff for inclusion. A 2-sided P value of <0.05 was considered statistically significant.
Results
A total of 1,933 patients underwent pancreatic resection for PDAC at Johns Hopkins Hospital from 2000 to 2016 and were included in the study. Approximately half (51.9%) were male with a median age of 67 (IQR, 59–75) at the time of operation. A history of hypertension was recorded in 40.4% of patients with a prescribed β-blocker documented in 23.4%. Of these, 392 (20.3%) were prescribed β1 selective, and 60 (3.1%) non-selective β-blockers. Additional detailed demographic and clinicopathologic characteristics for all included patients are summarized in Table 1.
Table 1
| Variable | Entire cohort (n=1,933) | Patients without β-blockers (n=1,476) | Patients on β-blockers (n=457) | P value* |
|---|---|---|---|---|
| Age (years), median (IQR) | 67 (59 to 75) | 66 (58 to 74) | 71 (64 to 77) | <0.001 |
| Male, n (%) | 1,003 (51.9) | 752 (51.0) | 251 (54.9) | 0.137 |
| Race | 1,633 (87.2) | 0.986 | ||
| Caucasian | 121 (6.5) | 1,246 (87.2) | 387 (87.2) | |
| Non-Caucasian | 119 (6.4) | 183 (12.8) | 57 (12.8) | |
| History of HTN, n (%) | <0.001 | |||
| No | 1,130 (59.6) | 961 (66.3) | 169 (37.9) | |
| Yes | 765 (40.4) | 488 (33.7) | 277 (62.1) | |
| Pain on presentation, n (%) | ||||
| No | 1,272 (68.6) | 970 (68.5) | 302 (68.8) | |
| Yes | 583 (31.4) | 446 (31.5) | 137 (31.2) | |
| Neoadjuvant treatment, n (%) | 329 (17.6) | 242 (17.0) | 87 (19.6) | 0.373 |
| Surgery type | 0.22 | |||
| Pancreaticoduodenectomy | 1,548 (80.1) | 1,187 (80.4) | 361 (79.0) | |
| Distal pancreatectomy | 294 (15.2) | 215 (14.6) | 79 (17.3) | |
| Total pancreatectomy | 91 (4.7) | 74 (5.0) | 17 (3.7) | |
| Tumor size (cm), median (IQR) | 3.1 (2.2–4.0) | 3.0 (2.2–4.0) | 3.0 (2.3–3.6) | 0.906 |
| T stage, n (%) | 0.312 | |||
| T1 | 201 (10.6) | 146 (10.1) | 55 (12.4) | |
| T2 | 428 (22.6) | 321 (22.1) | 107 (24.0) | |
| T3 | 1,210 (63.8) | 938 (64.6) | 272 (61.1) | |
| T4 | 58 (3.1) | 47 (3.2) | 11 (2.5) | |
| Nodal status, n (%) | 0.524 | |||
| N0 | 555 (28.8) | 419 (28.4) | 136 (30.0) | |
| N1 | 1,374 (71.2) | 1,056 (71.6) | 277 (70.0) | |
| Grade, n (%) | 0.938 | |||
| Well differentiated | 80 (4.3) | 62 (4.3) | 18 (4.1) | |
| Moderately differentiated | 1,023 (54.4) | 780 (54.2) | 243 (55.1) | |
| Poorly differentiated | 777 (41.3) | 597 (41.5) | 180 (40.8) | |
| Resection margin, n (%) | ||||
| R0 | 1,294 (67.6) | 985 (67.3) | 309 (68.7) | |
| R1 | 619 (32.4) | 478 (32.7) | 141 (31.3) | |
| PNI, n (%) | 0.101 | |||
| No | 286 (14.9) | 208 (14.2) | 78 (17.3) | |
| Yes | 1,630 (85.1) | 1,258 (85.8) | 372 (82.7) | |
| Lymphovascular invasion, n (%) | ||||
| No | 894 (47.7) | 685 (47.6) | 209 (48.1) | |
| Yes | 981 (52.3) | 755 (52.4) | 226 (51.9) | |
*, comparison of Patients without β-blockers and patients on β-blockers.
When compared to patients without a listed β-blocker prescription, patients taking β-blockers in the perioperative period were more likely to be older (71 vs. 66, P<0.001) and have a history of hypertension (62.1% vs. 33.7%, P<0.001). Tumor characteristics were not statistically significant when comparing these two groups: including similar tumor size, T stage, nodal status, grade, resection margin status and lymphovascular invasion. A non-significant trend was appreciated with fewer patients prescribed β-blockers had associated findings of PNI on histopathologic analysis (82.7% vs. 85.8%, P=0.101). This trend reached statistical significance when analyzed according to selectivity of β blockade. Non-selective β-blocker use was associated with significantly less PNI (nonselective β-blocker: 68.3%, β1 selective: 84.9%, No β-blocker: 85.9%, P=0.001). Additional detailed findings are summarized in Table 1.
PNI
Further analyses were performed with the presence of PNI set as the primary outcome to investigate the likelihood of a type I statistical error. On multivariate analysis a significantly lower rate of PNI was associated with neoadjuvant treatment (OR: 0.33; 95% CI: 0.23–0.48, P<0.001) and nonselective β-blocker use (OR: 0.36; 95% CI: 0.17–0.77, P=0.008). A higher T stage (OR: 2.39; 95% CI: 1.47–3.90, P<0.001), positive nodal status (OR: 1.86; 95% CI: 1.31–2.64, P=0.001), positive lymphovascular invasion (OR: 3.22; 95% CI: 2.22–4.68, P<0.001) and high grade (OR: 2.38; 95% CI: 1.26–4.48, P=0.007) were independently associated with significantly higher rates of PNI (Table 2).
Table 2
| Variable | PNI present (n=1,630) | PNI absent (n=286) | Univariate P value | OR | 95% CI | Multivariate P value |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| <65 | 593 (36.4) | 117 (40.9) | ref. | |||
| >65 | 1,037 (63.6) | 169 (59.1) | 0.144 | |||
| Male | 843 (51.7) | 150 (52.5) | 0.82 | |||
| Race | ||||||
| Caucasian | 1,385 (87.7) | 233 (84.4) | ref. | |||
| Other | 195 (12.3) | 43 (15.6) | 0.138 | |||
| History of HTN, n (%) | ||||||
| No | 940 (58.6) | 179 (65.6) | ref. | |||
| Yes | 665 (41.4) | 94 (34.4) | 0.029 | 1.41 | 0.99–1.98 | 0.052 |
| Pain on presentation, n (%) | ||||||
| No | 1069 (68.2) | 191 (70.7) | ref. | |||
| Yes | 499 (31.8) | 79 (29.3) | 0.402 | |||
| Neoadjuvant treatment, n (%) | 215 (13.6) | 108 (40.6) | <0.001 | 0.33 | 0.23–0.48 | <0.001 |
| Current Beta-blocker use | ||||||
| None | 1,264 (77.5) | 208 (72.7) | ref. | |||
| β1 selective | 326 (20.0) | 59 (20.6) | 0.265 | 0.8 | 0.54–1.19 | 0.265 |
| β1/β2 non-selective | 41 (2.5) | 19 (6.6) | <0.001 | 0.36 | 0.17–0.77 | 0.008 |
| Surgery type | ||||||
| Pancreaticoduodenectomy | 1,336 (82.0) | 198 (69.2) | ref. | |||
| Distal pancreatectomy | 219 (13.4) | 73 (25.5) | <0.001 | 0.77 | 0.51–1.18 | 0.227 |
| Total pancreatectomy | 75 (4.6) | 15 (5.2) | 0.306 | 0.82 | 0.39–1.76 | 0.615 |
| Tumor size | ||||||
| <3 cm | 696 (42.7) | 164 (57.3) | ref. | |||
| >3 cm | 934 (57.3) | 122 (42.7) | <0.001 | 1.11 | 0.78–1.59 | 0.551 |
| T stage, n (%) | ||||||
| T1 | 115 (7.1) | 85 (31.5) | ref. | |||
| T2 | 355 (22.0) | 71 (26.3) | <0.001 | 2.09 | 1.26–3.47 | 0.004 |
| T3 | 1,098 (67.9) | 105 (38.9) | <0.001 | 2.39 | 1.47–3.90 | <0.001 |
| T4 | 49 (3.0) | 9 (3.3) | <0.001 | 1.32 | 0.52–3.37 | 0.557 |
| Nodal status, n (%) | ||||||
| N0 | 379 (23.3) | 172 (60.4) | ref. | |||
| N1 | 1,250 (76.7) | 113 (39.6) | <0.001 | 1.86 | 1.31–2.64 | 0.001 |
| Grade, n (%) | ||||||
| Well differentiated | 57 (3.6) | 23 (9.0) | ref. | |||
| Moderately differentiated | 862 (53.5) | 156 (60.7) | 0.002 | 1.82 | 1.00–3.32 | 0.05 |
| Poorly differentiated | 691 (42.9) | 78 (30.4) | <0.001 | 2.38 | 1.26–4.48 | 0.007 |
| Resection margin, n (%) | ||||||
| R0 | 1,069 (66.0) | 219 (77.9) | ref. | |||
| R1 | 552 (34.0) | 62 (22.1) | <0.001 | 1.13 | 0.77–1.66 | 0.529 |
| Lymphovascular invasion, n (%) | ||||||
| No | 666 (41.8) | 228 (80.9) | ref. | |||
| Yes | 927 (58.2) | 54 (19.2) | <0.001 | 3.22 | 2.22–4.68 | <0.001 |
OS
Cox proportional-hazards regression model was performed to assess factors and characteristics independently associated with OS in resected PDAC patients with detail found in Table 3. As anticipated, patients with more advanced features and aggressive histopathologic findings had inferior survival. Notably poor prognostic indicators included increasing tumor size (OR: 1.12; 95% CI: 1.08–1.16, P<0.001), higher stage (OR: 1.50; 95% CI: 1.03–2.17, P=0.032), higher grade (OR: 2.04; 95% CI: 1.53–2.71, P<0.001), positive nodal metastases (OR: 1.39; 95% CI: 1.21–1.59, P<0.001), PNI (OR: 1.23; 95% CI: 1.02–1.47, P=0.028) and positive resection margin (OR: 1.44; 95% CI: 1.28–1.62, P<0.001). Interestingly, the receipt of neoadjuvant treatment was not significantly associated with OS in univariate analysis of this cohort. Nonselective β-blocker use was independently associated with significantly improved OS in this cohort (OR: 0.62; 95% CI: 0.44–0.88, P=0.007).
Table 3
| Variable | Univariate P value | HR | 95% CI | Multivariate P value |
|---|---|---|---|---|
| Age | <0.001 | 1.01 | 1.01–1.02 | <0.001 |
| Male | 0.753 | |||
| Race | ||||
| Caucasian | ref. | |||
| Other | 0.029 | 0.9 | 0.76–1.07 | 0.251 |
| History of HTN, n (%) | ||||
| No | ref. | |||
| Yes | 0.008 | 1.08 | 0.96–1.20 | 0.207 |
| Pain on presentation, n (%) | ||||
| No | ref. | |||
| Yes | 0.295 | |||
| Neoadjuvant treatment, n (%) | 0.8 | |||
| Current Beta-blocker use | ||||
| None | ref. | |||
| β1 selective | 0.107 | 1.06 | 0.93–1.21 | 0.4 |
| β1/β2 non-selective | 0.01 | 0.62 | 0.44–0.88 | 0.007 |
| Surgery type | ||||
| Pancreaticoduodenectomy | ref. | |||
| Distal pancreatectomy | 0.01 | 1.03 | 0.87–1.22 | 0.733 |
| Total pancreatectomy | 0.775 | 1.14 | 0.89–1.45 | 0.298 |
| Tumor size | <0.001 | 1.12 | 1.08–1.16 | <0.001 |
| T stage, n (%) | ||||
| T1 | ref. | |||
| T2 | <0.001 | 1.01 | 0.78–1.30 | 0.938 |
| T3 | <0.001 | 1.32 | 1.04–1.67 | 0.021 |
| T4 | <0.001 | 1.5 | 1.03–2.17 | 0.032 |
| Nodal status, n (%) | ||||
| N0 | ref. | |||
| N1 | <0.001 | 1.39 | 1.21–1.59 | <0.001 |
| Grade, n (%) | ||||
| Well differentiated | ref. | |||
| Moderately differentiated | 0.002 | 1.57 | 1.18–2.08 | 0.002 |
| Poorly differentiated | <0.001 | 2.04 | 1.53–2.71 | <0.001 |
| Resection margin, n (%) | ||||
| R0 | ref. | |||
| R1 | <0.001 | 1.44 | 1.28–1.62 | <0.001 |
| PNI, n (%) | ||||
| No | ref. | |||
| Yes | <0.001 | 1.23 | 1.02–1.47 | 0.028 |
| Lymphovascular invasion, n (%) | ||||
| No | ref. | |||
| Yes | <0.001 | 1.12 | 0.99–1.25 | 0.06 |
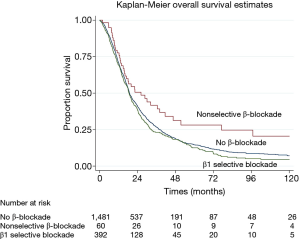
A median OS of 18.6 months was found for all patients. The administration of β1 selective blockade was not associated with an increase in OS (18.8 vs. 18.5 months, P=0.105), however, patients with nonselective β-blocker had significantly longer median OS compared to no β-blocker use (26.1 vs. 18.5 months, P=0.0016) (Figure 1).
Growth and invasion assays
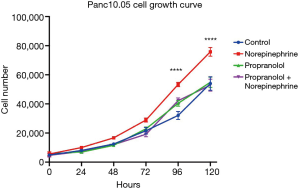
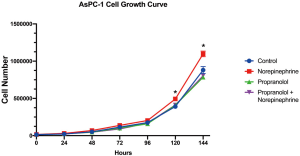
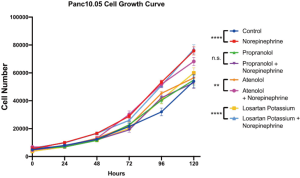
The significant associations with nonselective β-blocker use with PNI and OS prompted additional investigation of tumor biology in an in vitro setting. Panc10.05 growth was assessed with or without supplemental norepinephrine and propranolol. PDAC tumor growth was enhanced with a statistically significant increase of cell numbers at 96 (P<0.001) and 120 hours (P<0.001) with the addition of norepinephrine. However, norepinephrine did not significantly increase growth in the presence of propranolol. While propranolol alone did not decrease growth compared to untreated controls, the growth enhancement by norepinephrine was mitigated with nonselective β-blockade (Figure 2). These results were consistent in an additional human derived PDAC cell line: AsPC-1 (Figure S1). To further investigate the importance of non-selective β-blockade, the impact of norepinephrine on the growth of Panc10.05 was assessed in the presence of the β1-selective atenolol and an alternative antihypertensive, losartan.
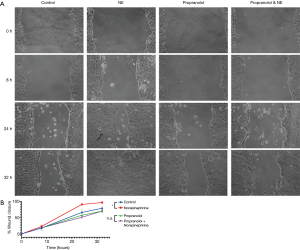
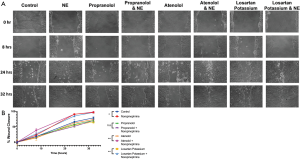
Cell migration scratch assay similarly revealed significantly enhanced invasiveness as represented by tumor cell motility at 24 hours (P<0.01) and 32 hours (P<0.05) in the cells supplemented with norepinephrine as compared to control treated Panc10.05 cells (Figure 3). This was again mitigated with the addition of propranolol with no significant difference in tumor cell motility comparing propranolol and the combination propranolol and norepinephrine (Figure 3). Notably, neither atenolol nor losartan recapitulated the mitigating effects of propranolol on norepinephrine with a significant increase in both growth and motility comparing treated cells (atenolol or losartan) to the combination of atenolol or losartan and norepinephrine (Figures S2,S3).
Discussion
Perioperative non-selective β-blocker use was independently associated with decreased PNI and improved OS in patients undergoing resection for PDAC. In vitro PDAC modeling of noradrenergic stimulation demonstrates increased cancer tumorgenicity that is abrogated with non-selective β-blockade. Together, these findings suggest a possible mechanism of action whereby enhanced local noradrenergic signaling within the PDAC TME may lead to increased tumorgenicity. This mechanism suggests that selective antagonism of noradrenergic signaling may play a role in mitigating the invasive phenotype of disease, a finding that is manifest in clinical data suggesting decreased PNI with the use of non-selective β- blockade. β-blockers may therefore be an intriguing therapeutic adjunct for PDAC.
PNI is appreciated in multiple tumor subtypes, however it is especially prevalent in PDAC reported in as high as 80–100% of cases (22,23). This histologic characteristic is universally associated with poorer prognosis and as anticipated, an independent predictor of poor survival in this study (6,7,22,23). The presence of PNI in this study, was significantly associated with general factors of more aggressive tumor biology including poor differentiation, lymphovascular invasion, lymph node metastasis and larger tumor size. Non-selective β-blockers use however, was an independent protective factor for PNI with a remarkable absolute difference of nearly 20%. Interestingly however, there was no correlation with non-selective β-blockers and other clinicopathologic features: patients had tumors of similar size, grade and stage regardless of β-blocker use, only PNI was affected. Renz et al. reported a trend of lower PNI rate in patients with pancreatic cancer taking nonspecific β-blockers that perhaps did not reach statistical significance due to a smaller number of patients than this present study (17). This highlights a mechanism of adrenergic signaling, potentially confined to the perineural TME, leading to this histopathologic phenomenon.
The adrenergic signaling pathway’s effect on cancer biology has been previously investigated, often motivated by observations of the impact of stress on increased cancer progression (9,24,25). β2-adrenergic signaling in particular, has been shown to have multiple potential downstream molecular effects including stimulating epithelial proliferation, triggering DNA damage, suppressing p53 levels, and increasing matrix metalloproteinase related tissue invasion and tumor cell motility (17,25-27). Previous published work reported β2-dependent PDAC promotion associated with increased pancreatic nerve density and upregulation of neurotrophins, while also identifying an epidemiologic relationship of nonselective β blocker use and improved OS (17). This present study supports these previously published findings, similarly finding longer OS associated with nonspecific β-blockade while utilizing a much larger patient series. The importance of the β2 pathway is further supported by the findings of this study; while individuals on non-selective β-blockers had decreased rates of PNI and improved OS, those with β1 selective blockade had no appreciated advantage.
Interestingly, neoadjuvant therapy was independently associated with less PNI but did not affect OS. Whether decreased PNI following neoadjuvant therapy is an association of the therapy received or of patient selection alone is unknown (i.e., those with aggressive tumors and PNI at diagnosis are more likely to progress on a neoadjuvant approach and fail to reach surgery). This study spans a large time period and treatment philosophies have changed as neoadjuvant therapy for locally advanced and borderline resectable PDAC has proven to be safe, efficacious and lead to encouraging survival (28-30). Previous reports have similarly shown PNI to be decreased following neoadjuvant treatment, although the survival benefit remains to be proven and is challenging to truly compare in retrospective studies (7). The combination of β-blockers and gemcitabine led to a significant survival advantage compared to gemcitabine alone in a preclinical mouse model of pancreatic cancer (17). Whether or not β-blocker use synergizes and augments the effect of modern neoadjuvant therapy and its interaction with PNI warrants future investigation.
These data are suggestive of progress being made in other academic cancer biology laboratories studying the oncologic effects of commonly used pharmaceuticals. Perhaps most striking is the similarity between this work and another antihypertensive class, the angiotensin II receptor blockers (ARBs). Epidemiologic studies have suggested improved survival following resection for PDAC in individuals taking ARBs (31). In advanced pancreatic cancer inhibition of angiotensin signaling has also been associated with improved prognosis, and has led to its use as an adjuvant to accepted therapies in multiple clinical trials (32,33). The effect of ARBs on the TME is an area of intense focus for several laboratories and the clinical effect of these agents is the focus on at least one ongoing clinical trial (NCT 03563248). Nevertheless, the effect of ARBs on the TME appears distinct from the adrenergic pathway as supported by a failure of losartan to abrogate norepinephrine induced growth or motility in this study (Figures S2,S3).
The findings of this work need to be viewed in the context of several limitations. Firstly, while this series represents nearly 2,000 consecutive patients with resected PDAC, only 60 patients were given nonselective beta blockers in the preoperative period. This rate of 3.1% is similar to previous reports of 2.7% in a series of 631 patients with pancreatic cancer (17). Nevertheless, to the authors’ knowledge this represents the largest report in literature. Although a prospective database from a large tertiary referral center was leveraged, the main clinical outcome of interest (survival) was collated and associated in a retrospective fashion, risking associated bias of retrospective work. Inevitably, accurate long-term follow-up for all patients can be challenging as many patients are referred to our tertiary center for resection. These patients typically receive their initial surveillance with the surgical providers for the first year, however are then frequently transitioned to follow-up by home institutions and may be lost to follow-up, only later to be identified by documented deaths. This follow-up limitation, however, is equally distributed across all groups and time frames. The categorization of β-blocker use was based upon documentation at the time of resection and data reliably determining patient treatment adherence to these home medications are unavailable. Similarly, it remains possible that not all individuals taking β-blockers were captured in the medical record at the time of patient evaluation. Additionally, the duration of β-blocker use was not readily available via our data abstraction methods. In our in vitro models, significant effects on tumor growth and motility after simultaneous norepinephrine and nonselective β-blocker treatment were noted after a period of only 1 to 3 days. In the future, it will be interesting to study if the timing or duration of clinical β-blocker treatment impacts the ability to modulate the TME. Next, our co-culture experiments did not include any additional cell types. The TME is complex and β-blockers may impact tumorgenicity through other cell types, such as endothelial cells as suggested in prostate cancer (11). Future studies including additional cell types to our co-culture are being pursued. Lastly, the in vitro effects of nonselective β-blockers were investigated without concurrent chemotherapy. The majority of patients in this study did not receive neoadjuvant chemotherapy, thus the observed modulating effects in the absence of chemotherapeutics are largely applicable, however future efforts investigating potential synergistic effects on tumor response of nonselective β-blockers with concomitant chemotherapy are of interest and relevance in the current era of PDAC management.
Conclusions
In conclusion, non-selective β-blocker use in patients with resected PDAC was associated with significantly lower rates of PNI and longer OS. PDAC patients continue to have a dismal survival and thus there is an urgent need for effective therapeutic approaches. β-blockers are readily available, inexpensive, safe and well-understood drugs. This study helps to provide additional rationale for future prospective studies investigating non-selective β-blockers as an adjuvant to existing chemotherapy regimens in PDAC patients.
Acknowledgments
This work was presented at the Society for Surgery of the Alimentary Tract Resident and Fellows Research Conference and Digestive Disease Week; May 2019; San Diego, CA, USA.
Funding: This work was supported by NIH grant T32 CA126607 (ABB and LZ).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-19-45). JLC serves as an unpaid editorial board member of Annals of Pancreatic Cancer from March 2017 to December 2020. CLW serves as an unpaid editorial board member of Annals of Pancreatic Cancer from March 2017 to December 2020. LZ serves as the Editor-in-Chief of Annals of Pancreatic Cancer and receives grant support from Bristol-Meyer Squibb, Merck, iTeos, Amgen, NovaRock, Inxmed, and Halozyme. LZ is a paid consultant/Advisory Board Member at Biosion, Alphamab, NovaRock, Akrevia, Datarevive, and Mingruzhiyao. LZ holds shares at Alphamab and Mingruzhiyao. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Johns Hopkins Hospital (No. 00074221), conducted in accordance with the Declaration of Helsinki. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318-48. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Groot VP, Gemenetzis G, Blair AB, et al. Implications of the Pattern of Disease Recurrence on Survival Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2018;25:2475-83. [Crossref] [PubMed]
- Zheng L, Xue J, Jaffee EM, et al. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013;144:1230-40. [Crossref] [PubMed]
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266-76. [Crossref] [PubMed]
- Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol 2012;36:409-17. [Crossref] [PubMed]
- Takahashi H, Ohigashi H, Ishikawa O, et al. Perineural invasion and lymph node involvement as indicators of surgical outcome and pattern of recurrence in the setting of preoperative gemcitabine-based chemoradiation therapy for resectable pancreatic cancer. Ann Surg 2012;255:95-102. [Crossref] [PubMed]
- Masur K, Niggemann B, Zanker KS, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 2001;61:2866-9. [PubMed]
- Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 2012;18:1201-6. [Crossref] [PubMed]
- Chang A, Kim-Fuchs C, Le CP, et al. Neural Regulation of Pancreatic Cancer: A Novel Target for Intervention. Cancers (Basel) 2015;7:1292-312. [Crossref] [PubMed]
- Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013;341:1236361. [Crossref] [PubMed]
- Zahalka AH, Arnal-Estape A, Maryanovich M, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017;358:321-6. [Crossref] [PubMed]
- Braadland PR, Ramberg H, Grytli HH, et al. beta-Adrenergic Receptor Signaling in Prostate Cancer. Front Oncol 2015;4:375. [PubMed]
- De Giorgi V, Grazzini M, Gandini S, et al. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 2011;171:779-81. [Crossref] [PubMed]
- Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011;29:2645-52. [Crossref] [PubMed]
- Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010;1:628-38. [Crossref] [PubMed]
- Renz BW, Takahashi R, Tanaka T, et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018;33:75-90 e7.
- Udumyan R, Montgomery S, Fang F, et al. Beta-Blocker Drug Use and Survival among Patients with Pancreatic Adenocarcinoma. Cancer Res 2017;77:3700-7. [Crossref] [PubMed]
- Rucki AA, Foley K, Zhang P, et al. Heterogeneous Stromal Signaling within the Tumor Microenvironment Controls the Metastasis of Pancreatic Cancer. Cancer Res 2017;77:41-52. [Crossref] [PubMed]
- Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993;90:3539-43. [Crossref] [PubMed]
- Guo K, Ma Q, Wang L, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep 2009;22:825-30. [PubMed]
- Hirai I, Kimura W, Ozawa K, et al. Perineural invasion in pancreatic cancer. Pancreas 2002;24:15-25. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006;6:240-8. [Crossref] [PubMed]
- Hara MR, Kovacs JJ, Whalen EJ, et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 2011;477:349-53. [Crossref] [PubMed]
- Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006;12:939-44. [Crossref] [PubMed]
- Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 2006;66:10357-64. [Crossref] [PubMed]
- Blair AB, Rosati LM, Rezaee N, et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: The impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery 2018;163:1090-6. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Javed AA, Wright MJ, Siddique A, et al. Outcome of Patients with Borderline Resectable Pancreatic Cancer in the Contemporary Era of Neoadjuvant Chemotherapy. J Gastrointest Surg 2019;23:112-21. [Crossref] [PubMed]
- Cerullo M, Gani F, Chen SY, et al. Impact of Angiotensin Receptor Blocker Use on Overall Survival Among Patients Undergoing Resection for Pancreatic Cancer. World J Surg 2017;41:2361-70. [Crossref] [PubMed]
- Nakai Y, Isayama H, Ijichi H, et al. Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer 2010;103:1644-8. [Crossref] [PubMed]
- Nakai Y, Isayama H, Ijichi H, et al. A multicenter phase II trial of gemcitabine and candesartan combination therapy in patients with advanced pancreatic cancer: GECA2. Invest New Drugs 2013;31:1294-9. [Crossref] [PubMed]
Cite this article as: Blair AB, Jurcak NR, Javed A, Teinor J, Groot VP, Rozich N, Cameron JL, Weiss MJ, He J, Wolfgang CL, Zheng L, Burkhart RA. Nonselective β-adrenergic blockade impacts pancreatic cancer tumor biology, decreases perineural invasion and improves patient survival. Ann Pancreat Cancer 2020;3:8.


