
Resected metachronous renal metastasis of pancreatic cancer after pancreaticoduodenectomy—a case report
Introduction
Pancreatic cancer harbors high malignant potential with high frequency of local invasion and distant metastasis, with the 5-year overall survival of approximately 7% in Japan (1). Even after curative pancreatectomy, most of them would experience metastasis, commonly to the liver, peritoneum or the lungs (2). We experienced a case of metachronous renal metastasis of pancreatic cancer treated with nephrectomy and adjuvant chemotherapy, who remained recurrent free for 10 months after metastasectomy. Renal metastasis of pancreatic cancer is extremely rare with a single case report of a synchronous metastasis existing among the literatures (3). Although treatment centers on non-surgical strategies for advanced staged diseases, reports advocating benefits of resection of metastatic lesions are increasing (4-6). This is an initial report of a patient who underwent resection of a solitary renal metastasis from pancreatic cancer. In selected patients, surgical treatment for metastatic lesion may play a favorable role in prolonging survival.
Case presentation
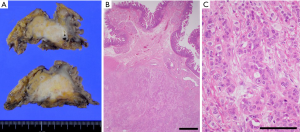
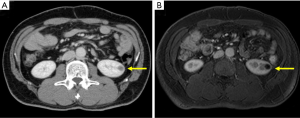
A 58-year-old otherwise healthy man diagnosed with pancreatic head cancer underwent pancreaticoduodenectomy with portal vein resection. Preoperative contrast enhanced computed tomography (CT) showed a 25 mm hypovascular lesion at the pancreatic head with invasion to the superior mesenteric vein. Lymph nodes were not enlarged, and no distant metastases were detected. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were elevated at 12.3 µg/L (reference range: 0.8–4.8) and 129 U/mL, (reference range: <36) respectively. Splenic vein was preserved and complete microscopic resection (R0) was achieved. The pathological results were adenocarcinoma with squamous differentiation (Figure 1), pT3N1bM0 stage IIB according to the 7th edition of Japanese General Rules for the Study of Pancreatic Cancer (7) (T2N2M0 stage III in UICC classification). Adjuvant chemotherapy with S1 (tegafur, gimeracil, oteracil) was initiated according to the “Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis” (8). Follow up CT examined 9 months after surgery revealed a 10 mm hypovascular nodule in the left kidney (Figure 2A). As there were no elevation of the tumor markers, primary renal tumor or focal bacterial nephritis was suspected. Magnetic resonance imaging 2 months later revealed growth of the nodule to 15 mm (Figure 2B), and left nephrectomy was indicated for suspected primary renal tumor.
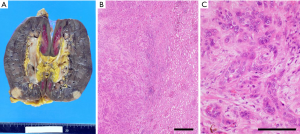
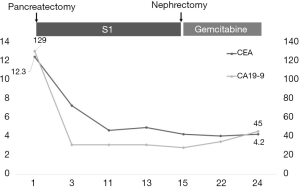
Immunohistochemistry results of the resected tumor were CAIX(–), AMACR(–), and PAX8(±), indicating that the tumor was not renal origin. Pathological results were compatible with adenocarcinoma of pancreatic origin (Figure 3), and we initiated adjuvant chemotherapy with gemcitabine. He is routinely followed up, and from the tumor markers and imaging studies, there is no definite evidence of recurrence for 10 months (Figure 4).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
This case highlighted two important issues. First, this is the first report of a resected renal metastasis of pancreatic cancer. Second, metastasectomy for pancreatic cancer should be considered in selected cases of oligometastatic disease after stability on chemotherapy.
Renal metastases of pancreatic cancers were identified in the autopsy series (9), however, there was only one previous case report of a pathologically diagnosed pancreatic cancer metastasizing to the kidney, which was a synchronous metastasis (3). Pancreatic cancer aggressively invades the local structures and metastasize to the distant organs, such as lung and liver in a short period. For this patient, as the lymph node metastasis was positive at primary resection, microscopic hematogenous metastasis may have taken place. We hypothesize that adjuvant S1 therapy favorably treated the microscopic metastasis for about 9 months. Thereafter, uncontrollable cancer increased solitary at the kidney.
This patient remained recurrent free for about 10 months after nephrectomy which was about 2 years after primary resection. While the median survival period after recurrence of pancreatic ductal adenocarcinoma was reported to be about 7 months (10), reports focusing on the significance of metastatic resection of pancreatic cancers are increasing. Groot et al. reported an overall survival of 69 months for the 19 patients operated for metachronous solitary lung metastases (4). Dünschede et al. reported an overall survival of 31 months for the 4 patients operated on metachronous liver metastasis (6). However, Chang et al. reported a possible survival benefit of reoperation only for non-adenocarcinoma recurrences (5). As these studies had limited population, the efficacy of resection of metastasis is still unclear and controversial; however, under effective control of chemotherapy, resection of metachronous solitary metastasis could be feasible and may contribute to improved survival. Further investigation is needed to clarify the prognostic factors associated with the efficacy of chemotherapy and repeated surgery, with special focus on genetic variants of individuals.
This is an initial report of a patient who underwent resection of a solitary renal metastasis from pancreatic cancer. As for the rarity of a solitary renal metastasis of pancreatic cancer, preoperative diagnosis could be difficult. When a renal mass is newly detected in the post-operative course of pancreatic cancer, renal metastasis should be suspected. Role of surgery for recurrent pancreatic cancer remains uncertain; however, aggressive resection of metastasis may contribute to better survival in selected patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-20-1). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Monitoring of Cancer Incidence in Japan - Survival 2006-2008 Report (Center for Cancer Control and Information Services NCC, 2016). Available online: https://ganjoho.jp/en/professional/statistics/table_download.html
- Renz BW, Boeck S, Roeder F, et al. Oligometastatic disease in pancreatic cancer - how to proceed? Visc Med 2017;33:36-41. [Crossref] [PubMed]
- Malkoç E, Aktaş Z, Kara K, et al. Renal metastasis from pancreatic adenocarcinoma: a rare case along with literature review. Turk J Urol 2015;41:93-5. [Crossref] [PubMed]
- Groot VP, Blair AB, Gemenetzis G, et al. Isolated pulmonary recurrence after resection of pancreatic cancer: the effect of patient factors and treatment modalities on survival. HPB (Oxford) 2019;21:998-1008. [Crossref] [PubMed]
- Chang SC, Hsu CP, Tsai CY, et al. Selective reoperation after primary resection as a feasible and safe treatment strategy for recurrent pancreatic cancer. Medicine (Baltimore) 2016;95:e4191 [Crossref] [PubMed]
- Dünschede F, Will L, von Langsdorf C, et al. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res 2010;44:209-13. [Crossref] [PubMed]
- Japan Pancreas Society. Classification of Pancreatic Carcinoma (4th English edition). 2017. Available online: http://www.suizou.org/pdf/Classification_of_Pancreatic_Carcinoma_4th_Engl_ed.pdf
- Okusaka T, Nakamura M, Yoshida M, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas 2020;49:326-35. [Crossref] [PubMed]
- Kamisawa T, Isawa T, Koike M, et al. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 1995;11:345-9. [Crossref] [PubMed]
- Gbolahan OB, Tong Y, Sehdev A, et al. Overall survival of patients with recurrent pancreatic cancer treated with systemic therapy: a retrospective study. BMC Cancer 2019;19:468. [Crossref] [PubMed]
Cite this article as: Igata Y, Kobayashi Y, Okubo S, Shindoh J, Hashimoto M. Resected metachronous renal metastasis of pancreatic cancer after pancreaticoduodenectomy—a case report. Ann Pancreat Cancer 2020;3:9.


