Fluoropyrimidine-based maintenance chemotherapy after FOLFIRINOX for patients with metastatic pancreatic adenocarcinoma: a retrospective analysis
Introduction
FOLFIRINOX was the first chemotherapy regimen to show improved outcomes when compared to single-agent gemcitabine in the first-line treatment of metastatic pancreatic cancer. In the pivotal PRODIGE 4/ACCORD 11 study, treatment with FOLFIRINOX led to improvements in overall response rate (31.6% vs. 9.4%), progression-free survival (PFS) (median: 6.4 vs. 3.3 months) and overall survival (OS) (median: 11.1 vs. 6.8 months) (1). As a consequence, FOLFIRINOX became one the most commonly used chemotherapy regimens in the treatment of advanced pancreatic cancer (2,3).
However, FOLFIRINOX is associated with significant toxicities. Grades 3/4 neutropenia, diarrhea, and peripheral neuropathy were reported to occur in 46%, 13%, and 9% of the patients, respectively (1). The incidence of severe diarrhea is increased among patients with polymorphisms involving UGT-1A1 (4), and dose reductions or even discontinuation of Irinotecan are often needed in these cases. Furthermore, some toxicities, such as the oxaliplatin-related peripheral neuropathy, are related to the total cumulative dose of the drug (5). As a result, these toxicities often hinder the continuation of the full FOLFIRINOX regimen, even in cases where it has proved to be significantly active against the tumor.
Currently, the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib is the only maintenance treatment approved for patients with metastatic pancreatic cancer. However, the activity of these drug has been attested only in the selected group of patients with germline mutations in BRCA-1/2 genes (4–7% of all patients) (6). Therefore, current evidence support the vast majority of patients derive no benefit from PARP inhibition.
Thus, apart from patients with germline mutations in BRCA1/2 genes, there is no standard approach to treatment de-escalation for patients experiencing significant toxicity during the treatment with FOLFIRINOX. We sought to describe the survival outcomes and toxicities of patients treated with first-line FOLFIRINOX that went on to receive 5-fluorouracil-based maintenance chemotherapy. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apc-20-27).
Methods
This is a retrospective, single-center study carried out in a cancer-specialized hospital. It was based on routinely collected data retrieved from the electronic charts of patients with metastatic pancreatic adenocarcinoma submitted to maintenance 5-fluorouracil-based chemotherapy after FOLFIRINOX. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the A.C. Camargo Cancer Center Internal Ethics Review Board (CAAE 822894.5.0000.5432).
Patients
We included patients aged 18 years or older diagnosed with pathologically confirmed pancreatic adenocarcinoma from Jan 1st 2010 to December 31th 2018, with ECOG (Eastern Cooperative Oncology Group) performance status 0–2, who underwent treatment with FOLFIRINOX for metastatic disease in the first-line setting, and received maintenance chemotherapy after at least four cycles of FOLFIRINOX with no progression during FOLFIRINOX. We excluded patients who received FOLFIRINOX out of the A.C. Camargo Cancer Center and those who had no response evaluation during treatment with FOLFIRINOX.
Treatment
We considered maintenance chemotherapy treatment with any fluoropyrimidine-based chemotherapy containing one or two drugs: FOLFIRI, FOLFOX, 5-fluorouracil, or capecitabine. Patients could have received full or attenuated doses of the aforementioned chemotherapy regimens.
Variables
We collected data on the following patients’ characteristics at the start of maintenance chemotherapy: age, gender, ECOG (Eastern Cooperative Oncology Group) performance status, Age-Adjusted Charlson Comorbidity Score (AACCS), tumor site (head/neck vs. body/tail), number of metastatic sites, serum CA 19-9 before the start of maintenance chemotherapy, number of FOLFIRINOX cycles before start of maintenance chemotherapy, type of first-line FOLFIRINOX (standard vs. modified), and reason to de-intensify treatment. We also gathered information on the following treatment features: type of maintenance chemotherapy, number of maintenance chemotherapy cycles, use of Granulocyte Colony-Stimulating Factors (G-CSF) during maintenance chemotherapy, and further lines of treatment, including re-challenge with FOLFIRINOX. Finally, we assembled data regarding grades 3/4 or severe toxicities. Patients for whom performance status data were not available had their performance inferred from the descriptions of patients’ capabilities found in the medical records.
Outcomes
The study primary outcome was PFS. Secondary outcomes were the OS from the start of maintenance chemotherapy (OS), the OS from the start of FOLFIRINOX (OS-FFX), and the toxicity profile. PFS was defined as the time from the start of maintenance chemotherapy to death or disease progression. Treatment response evaluations were carried out every 8 to 12 weeks with either computed tomography (CT) or magnetic resonance imaging (MRI). Disease progression was established after reviewing patients’ radiological reports and/or the clinical impressions from the treating physicians recorded in the medical chart. OS from maintenance was defined as the time from the start of maintenance chemotherapy to death from any cause. OS from the start of FOLFIRINOX was defined as the time from the start of FOLFIRINOX to death from any cause. Toxicity was graded according to the Common Toxicity Criteria for Adverse Events version 4.0. The toxicity analysis included the assessment of specific grades 3/4 toxicities, severe treatment-related toxicity (treatment-related complication mandating hospital admission) and treatment-related mortality. In an exploratory analysis, we analyzed whether there were differences in survival outcomes according to the maintenance chemotherapy regimen (FOLFOX vs. FOLFIRI).
Statistical analysis
The distributions of categorical variables were described using relative and absolute frequencies and the distributions of numerical variables were described using median values and interquartile ranges (IQR). Time-to-event variables (PFS, OS, and OS-FFX) were estimated by the Kaplan-Meier method and survival curves were compared using the log-rank test. We used two-tailed statistical tests and statistical analyses were performed with Stata Version 16 (StataCorp, College Station, Texas, USA).
Results
Overall, one hundred fifty-one patients with metastatic pancreatic adenocarcinoma were treated with FOLFIRINOX as first-line treatment at A.C. Camargo Cancer Center from 2010 to 2018. Ninety-six patients were excluded for the following reasons: FOLFIRINOX infusions out of A.C. Camargo Cancer Center (10 patients), progressive disease as best response to FOLFIRINOX (19 patients), no radiological response evaluation (22 patients), less than four cycles of FOLFIRINOX (six patients), and lack of maintenance chemotherapy after FOLFIRINOX (39 patients). Thus, the study population consists of 55 patients.
Median age at start of maintenance chemotherapy was 60 years (IQR: 55–65) and males were slightly more represented (N=29; 52.7%) (Table 1). Forty patients (72.7%) presented ECOG 0 at start of maintenance chemotherapy. Median age-adjusted Charlson comorbidity score was 8 (IQR: 7–9). Median CA 19-9 levels at start of maintenance chemotherapy was 88.2 UI/mL (IQR: 19.9–536.0 UI/mL) and most patients presented a single site of metastatic disease (N=34; 61.8%). Prior to start of maintenance chemotherapy, patients were submitted to a median of 10 cycles of FOLFIRINOX (IQR: 7–11) and 50.9% (N=28) of patients were treated with standard FOLFIRINOX. The most common reason for treatment de-escalation was peripheral neuropathy (N=26; 47.3%).
Table 1
| Characteristic | Number |
|---|---|
| Age (years) | |
| Median | 60 |
| Range | 42–78 |
| IQR | 55–65 |
| Gender, n (%) | |
| Male | 29 (52.7) |
| Female | 26 (47.3) |
| Performance status, n (%) | |
| ECOG 0 | 40 (72.7) |
| ECOG 1 | 15 (27.3) |
| Age-adjusted Charlson Comorbidity Score | |
| Median | 8 |
| IQR | 7–9 |
| Primary tumor site, n (%) | |
| Head or neck | 26 (47.3) |
| Body or tail | 29 (52.7) |
| CA 19-9 at de-escalation (U/mL) | |
| Median | 88.2 |
| IQR | 19.9–536.0 |
| Number of metastatic sites, n (%) | |
| 1 | 34 (61.8) |
| 2 | 15 (27.3) |
| ≥3 | 6 (10.9) |
| Number of FOLFIRINOX cycles prior to de-escalation | |
| Median | 10 |
| IQR | 7–11 |
| Type of FOLFIRINOX, n (%) | |
| Standard | 28 (50.9) |
| Modified | 27 (49.1) |
| Reason for treatment de-escalation$, n (%) | |
| Peripheral neuropathy | 26 (47.4) |
| Hematological toxicity | 12 (21.8) |
| Diarrhea | 6 (10.1) |
| Fatigue | 4 (7.3) |
| Others | 11 (20.0) |
| Unknown | 1 (2.0) |
$Patients can have more than one reason to de-escalate treatment.
Maintenance treatment
FOLFIRI was the most commonly used maintenance chemotherapy regimen (N=42; 76.4%) (Table 2). Excluding the only patient that started maintenance chemotherapy with single-agent 5-fluorouracil, 14.8% (N=8) of patients had further treatment de-escalation to single-agent 5-fluorouracil. Patients received a median of 8 cycles of maintenance chemotherapy (IQR: 4–15). Primary prophylaxis with G-CSF was used in 47.3% (N=26) of patients.
Table 2
| Characteristic | Number |
|---|---|
| Type of treatment, n (%) | |
| FOLFIRI | 42 (76.4) |
| FOLFOX | 12 (21.8) |
| 5-fluorouracil | 1 (1.8) |
| Further de-escalation to fluoropyrimidine monotherapy$, n (%) | |
| Yes | 8 (14.8) |
| No | 46 (85.2) |
| Granulocyte colony-stimulating factor, n (%) | |
| Yes—primary prophylaxis | 26 (47.3) |
| Yes—secondary prophylaxis | 3 (5.5) |
| No | 26 (47.3) |
| Number of cycles of chemotherapy after de-escalation | |
| Median | 8 |
| IQR | 4–15 |
$One patient excluded (direct de-escalation to 5-fluorouracil).
Survival
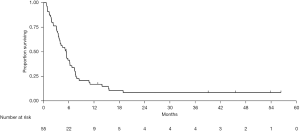
Median follow-up from start of maintenance chemotherapy was 45.5 months [95% confidence interval (95% CI), 22.6–NA]. Forty-nine patients experienced either disease progression or death from any cause. Median PFS was 5.4 months (95% CI, 3.7–6.3) (Figure 1). At three, six, and 12 months, 76.4%, 41.5%, and 17.0% were free of progression, respectively. In an exploratory analysis, there was no difference in median PFS between patients treated with FOLFOX or FOLFIRI as maintenance chemotherapy (FOLFOX: 6.2 months, 95% CI, 1.9–7.4; FOLFIRI: 5.2 months, 95% CI, 3.6–6.3; P=0.98).
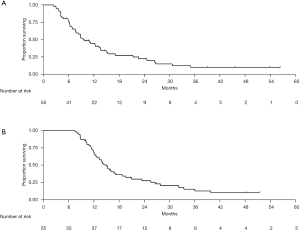
Forty-five patients died during follow-up. Median OS from the start of maintenance chemotherapy was 9.7 months (95% CI, 7.6–13.6) (Figure 2A). At 6, 12, and 18 months from start of maintenance chemotherapy, 76.3%, 44.3%, and 26.2% were alive, respectively. Median OS from the start of FOLFIRINOX was 15.0 months (95% CI, 12.4–17.1) (Figure 2B). At 12, 18, and 24 months from start of FOLFIRINOX, 68.7%, 36.3%, and 27.5% were alive, respectively. In an exploratory analysis, there was no difference in median OS from the start of maintenance chemotherapy between patients treated with FOLFOX (13.2 months, 95% CI, 8.8–NA) and those treated with FOLFIRI (8.4 months, 95% CI, 6.3–13.7; P=0.73). Likewise, there was no difference in median OS-FFX according to the maintenance chemotherapy regimen (FOLFOX: 16.7 months, 95% CI, 11.4–25.5; FOLFIRI: 13.9 months, 95% CI, 11.9–18.7; P=0.91).
Further lines of treatment
The median number of lines of chemotherapy was 2 (IQR: 2–3). Forty-four patients (80.0%) underwent second-line treatment, mainly with gemcitabine monotherapy (N=18; 40.9%). Among these patients, eighteen (40.9%) received a third line of treatment (Table 3). Re-challenge with FOLFIRINOX was performed in two (3.6%) patients.
Table 3
| Treatment | N (%) |
|---|---|
| Second-line treatment (N=55) | |
| Yes | 44 (80.0) |
| No | 11 (20.0) |
| Type of second-line treatment (N=44) | |
| Gemcitabine monotherapy | 18 (40.9) |
| Gemcitabine plus platinum | 3 (6.8) |
| Gemcitabine plus nab-paclitaxel | 5 (11.4) |
| FOLFOX | 11 (25.0) |
| FOLFIRI | 6 (13.6) |
| FOLFIRINOX | 1 (2.3) |
| Third-line treatment (N=44) | |
| Yes | 18 (40.9) |
| No | 26 (59.1) |
| Type of third-line treatment (N=18) | |
| Gemcitabine monotherapy | 6 (33.3) |
| Gemcitabine plus platinum | 0 (0.0) |
| Gemcitabine plus nab-paclitaxel | 3 (16.7) |
| FOLFOX | 2 (11.1) |
| FOLFIRI | 3 (16.7) |
| FOLFIRINOX | 1 (5.6) |
| Taxane | 2 (11.1) |
| Others | 1 (5.6) |
| Re-challenge with FOLFIRINOX (N=55) | |
| Yes | 2 (3.6) |
| No | 53 (96.4) |
Toxicity
Severe toxicities occurred in 15 patients (27.3%) during maintenance chemotherapy. The most common severe toxicity was severe sepsis/septic shock (11 patients; 73.3%) and intra-abdominal infections represented the most frequent cause of severe sepsis or septic shock (eight patients; 72.7%). Three patients (5.5%) died during maintenance toxicity. A 73-year-old former smoker male with systemic hypertension and diabetes mellitus died from adult respiratory distress syndrome after the treatment of severe sepsis, despite extensive investigation of the causes of respiratory failure. A 62-year-old male died as a consequence of severe sepsis/septic shock (abdominal site) in the context of fulminant disease progression. Last, a 61-year-old male died from cardiogenic shock secondary to non-ST elevated acute coronary syndrome and tumor-related constrictive pericarditis. The most common grade 3/4 toxicities experienced during maintenance chemotherapy were fatigue (N=6; 10.9%), neutropenia (N=8; 14.6%), and thrombocytopenia (N=9; 16.4%) (Table 4). Six patients (10.9%) evolved to grades 3/4 peripheral neuropathy during maintenance chemotherapy.
Table 4
| Toxicity | N (%) |
|---|---|
| Nausea | 0 (0.0) |
| Vomiting | 0 (0.0) |
| Constipation | 0 (0.0) |
| Diarrhea | 2 (3.6) |
| Mucositis | 0 (0.0) |
| Fatigue | 6 (10.9) |
| Anorexia | 1 (1.8) |
| Anemia | 2 (3.6) |
| Neutropenia | 8 (14.6) |
| Thrombocytopenia | 9 (16.4) |
| Febrile neutropenia | 2 (3.6) |
| Renal dysfunction | 4 (7.3) |
| Peripheral neuropathy | 6 (10.9) |
Discussion
FOLFIRINOX was proven to be one of the most significant developments in the treatment of advanced pancreatic cancer in the past decade. However, one of the challenges of using this chemotherapy regimen is dealing with long-term toxicity. With time, patients might experience significant side effects, such as peripheral neuropathy and diarrhea, that compel physicians to reduce the dose or to withhold oxaliplatin, irinotecan, or both drugs. In this study we report encouraging survival outcomes after treatment de-escalation, mostly to FOLFIRI, for patients with metastatic pancreatic cancer patients submitted to at least four cycles of FOLFIRINOX with no disease progression.
So far, there are very limited data from randomized trials to support the role of maintenance therapy in advanced pancreatic cancer. After first-line treatment (mostly combination chemotherapy), Sunitinib was associated with a modest improvement in median PFS (3.2 vs. 2.0 months; HR =0.51; P<0.01) when compared to observation in a small phase II randomized trial (7). Notably 22.2% of patients treated with sunitinib were progression-free at 6 months (vs. 3.6% for patients enrolled onto the observation arm). However, there was no difference in OS between treatment arms. Recently, the POLO trial assessed the role of maintenance olaparib, a poly-ADP ribose polymerase (PARP) inhibitor, in the very selected group of patients with germ-line mutation in either BRCA-1 or BRCA-2 genes (6). Olaparib was associated a significant improvement in PFS when compared to observation (7.4 vs. 3.8 months; HR =0.53; P=0.004). At the time of the publication, the OS curves were not considered mature; however, to that point, there was no evidence that olaparib was associated with improved survival, despite the relatively low crossover rate (14.5%) (8). Thus, maintenance treatment using targeted therapy has had some success in the treatment of advanced pancreatic cancer as maintenance therapy, but results are either modest or applicable to a very selected group of patients. Moreover, these drugs have not been approved for the treatment of pancreatic cancer in many countries, hampering their use in the clinical practice for many patients.
Alternatively, one approach to maintenance treatment that is reasonable is to use a de-escalated form of FOLFIRINOX. In clinical practice, maintenance with single-agent fluoropyrimidine or FOLFIRI is frequently used. However, there is very limited evidence to support the continuation of chemotherapy after 8 to 12 cycles of FOLFIRINOX in the first-line setting. In this sense, PRODIGE 35 (PANOPTIMOX) is the only randomized trial available so far that addressed the potential benefits of maintenance chemotherapy in metastatic pancreatic cancer (9). This was a three-arm non-comparative study evaluating the following strategies: FOLFIRINOX for 12 cycles followed by treatment interruption, FOLFIRINOX for 8 cycles followed by maintenance 5-fluorouracil, and sequential treatment with gemcitabine and FOLFIRI.3. There was no numerical difference in median PFS between the two treatment arms that used FOLFIRINOX (6.3 vs. 5.7 months). Also, there was no difference between the median OS of patients that underwent observation or maintenance 5-fluorouracil (10.1 vs. 11.0 months). However, patients treated with maintenance chemotherapy were more likely to survive for at least 18 months (28% vs. 18.5%). As a consequence, it seems that a subgroup of patients might benefit from maintenance chemotherapy after FOLFIRINOX.
Retrospective studies have shown that maintenance chemotherapy with FOLFIRI or single-agent capecitabine can be associated long PFS times and manageable toxicities. Franck et al. treated 22 patients with FOLFIRI after a median of four months on FOLFIRINOX (10). Median PFS was 8 months and grades 2 or more toxicities were infrequent. Reure et al. reported a median PFS of 5.0 months and a median OS from the start of FOLFIRINOX of 17.0 months when maintenance with capecitabine was used (11). Again, apart from hand-foot skin reaction, the toxicity profile was considered mild. Importantly, some studies have shown that re-challenge with FOLFIRINOX is feasible in a group of patients after disease progression on maintenance chemotherapy. In a small retrospective study by Hann et al., thirteen patients were treated with induction FOLFIRINOX followed by maintenance 5-fluorouracil (12). Median PFS and median OS from the start of FOLFIRINOX were 10.6 and 18.0 months, respectively. Importantly, eleven patients were able to resume FOLFIRINOX once they experienced progressive disease. While attention must be paid given the small number of patients in each of these studies, these results highlight the feasibility of this approach, both in terms of effectiveness and safety.
In our study, PFS was similar to the one found in the study by Reure et al. with maintenance capecitabine. Our results in this regard are inferior to the ones from the study by Franck et al. that used FOLFIRI as maintenance chemotherapy. However, care must be taken when analyzing these data as the abovementioned studies have a smaller sample size. Accordingly, our rates of re-challenge with FOLFIRINOX are much less than the ones reported by these studies. In our experience, re-challenge with FOLFIRINOX after pancreatic cancer progression in first-line is often difficult given the frequently observed deterioration of performance status. Additionally, many of our patients experienced disease progression while they were on FOLFIRI, suggesting that the simple addition of oxaliplatin was unlikely to control the disease and prompting the use of other regimens, such gemcitabine-based chemotherapy. That said, sample sizes and patient selection might explain differences is survival outcomes between our study and the previous ones.
In our study, three patients died during maintenance chemotherapy. However, in only one case death was considered to be possibly related to chemotherapy. In the other two cases, deaths were considered to be a consequence of disease progression. Nonetheless, severe toxicities occurred in a quarter of the patients. This is remarkable since most patients in our study presented ECOG 0 at the start of maintenance chemotherapy. Also, many of our patients were treated with primary G-CSF prophylaxis. Therefore, we believe this fact highlights the need to carefully select patients for maintenance chemotherapy.
Our study has limitations. First, it is a single center retrospective study. Second, we could not gather data on radiological response evaluation prior to or during maintenance chemotherapy. Last, the doses of each chemotherapy regimen during maintenance were not completely homogeneous. However, our study is one of the largest studies evaluating the role of maintenance chemotherapy after FOLFIRINOX for patients with metastatic pancreatic cancer. Also, we provide detailed information regarding the survival outcomes and the toxicity profiles of this treatment strategy.
To conclude, maintenance chemotherapy with 5-fluororuacil based chemotherapy is feasible and can be associated with lasting disease control in a subset of patients with metastatic pancreatic cancer. However, severe toxicities, associated with either chemotherapy or disease progression, can occur, making proper patient selection paramount to ascertain that patients derive the maximum benefit from this treatment strategy. More work is needed to find out the optimal chemotherapy maintenance protocol (single-agent fluoropyrimidine or fluoropyrimidine-based doublet) and the role of newer drugs as maintenance therapy for advanced pancreatic cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apc-20-27
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apc-20-27
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-20-27). VHF de Jesus received honoraria from United Medical and had travel expenses paid by United Medical in the past 12 months. United Medical is the company responsible for the distribution and sale of Nab-Paclitaxel in Brazil. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the A.C. Camargo Cancer Center Internal Ethics Review Board (CAAE 822894.5.0000.5432) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Abrams TA, Meyer G, Meyerhardt JA, et al. Patterns of Chemotherapy Use in a U.S.-Based Cohort of Patients with Metastatic Pancreatic Cancer. Oncologist 2017;22:925-33. [Crossref] [PubMed]
- Le N, Vinci A, Schober M, et al. Real-World Clinical Practice of Intensified Chemotherapies for Metastatic Pancreatic Cancer: Results from a Pan-European Questionnaire Study. Digestion 2016;94:222-9. [Crossref] [PubMed]
- Shirasu H, Todaka A, Omae K, et al. Impact of UGT1A1 genetic polymorphism on toxicity in unresectable pancreatic cancer patients undergoing FOLFIRINOX. Cancer Sci 2019;110:707-16. [Crossref] [PubMed]
- Argyriou AA. Updates on Oxaliplatin-Induced Peripheral Neurotoxicity (OXAIPN). Toxics 2015;3:187-97. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]
- Reni M, Cereda S, Milella M, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer 2013;49:3609-15. [Crossref] [PubMed]
- Kindler HL, Hammel P, Reni M, et al. Olaparib as maintenance treatment following first-line platinum-based chemotherapy (PBC) in patients (pts) with a germline BRCA mutation and metastatic pancreatic cancer (mPC): Phase III POLO trial. J Clin Oncol 2019;37:abstract LBA4.
- Dahan L, Phelip JM, Le Malicot K, et al. FOLFIRINOX until progression, FOLFIRINOX with maintenance treatment, or sequential treatment with gemcitabine and FOLFIRI.3 for first-line treatment of metastatic pancreatic cancer: A randomized phase II trial (PRODIGE 35-PANOPTIMOX). J Clin Oncol 2018;36. abstract 4000.
- Franck C, Canbay A, Malfertheiner P, et al. Maintenance Therapy with FOLFIRI after FOLFIRINOX for Advanced Pancreatic Ductal Adenocarcinoma: A Retrospective Single-Center Analysis. J Oncol 2019;2019:5832309 [Crossref] [PubMed]
- Reure J, Follana P, Gal J, et al. Effectiveness and Tolerability of Maintenance Capecitabine Administrated to Patients with Metastatic Pancreatic Cancer Treated with First-Line FOLFIRINOX. Oncology 2016;90:261-6. [Crossref] [PubMed]
- Hann A, Bohle W, Egger J, et al. Feasibility of alternating induction and maintenance chemotherapy in pancreatic cancer. Sci Rep 2017;7:41549. [Crossref] [PubMed]
Cite this article as: de Almeida MJ, de Mello CAL, Camandaroba MPG, de Jesus VHF. Fluoropyrimidine-based maintenance chemotherapy after FOLFIRINOX for patients with metastatic pancreatic adenocarcinoma: a retrospective analysis. Ann Pancreat Cancer 2020;3:13.