
Quantitative metric for assessment of pancreatic ductal adenocarcinoma treatment response in T1-weighted gadolinium-enhanced magnetic resonance imaging
Introduction
Pancreatic ductal adenocarcinomas (PDAs) are highly lethal cancers for which advances in detection and treatment are greatly needed. Although progress has been made in treatment and outcomes of other major cancers, the lack of early detection and relatively ineffective treatment of PDA, in addition to an apparent increasing incidence, are factors considered in predicting that PDA will be the 2nd leading cause of cancer death in the US by 2030 (1). Medical imaging plays an important role in evaluating PDA treatment response, and continued innovations in imaging are vital for support of trials validating new and improved treatments.
Current methods of assessing treatment response by imaging size measurements and serum tumor biomarkers levels have notable shortcomings in application to pancreatic cancer. Most imaging criteria for evaluating treatment response are based on size [e.g., World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) Guidelines (2)]. Computed tomography (CT) is the most often used modality to assess PDA, but cannot distinguish tumor margins or residual tumor from fibroinflammatory tissue due to limitations in soft-tissue contrast (3-6), which may adversely impact accuracy of size measurements. When changes in size are equivocal, serum tumor biomarkers can provide a surrogate measure of treatment response. Cancer antigen 19-9 (CA 19-9) is a widely used PDA biomarker, but it can be falsely elevated by benign conditions such as biliary obstruction, pancreatitis, colon cancer, or chronic liver disease, and is not elevated in 5–10% of PDAs (7).
Measurements of longitudinal relaxation time (T1) in magnetic resonance imaging (MRI) have been shown to decrease with treatment response in mouse models of neuroblastoma (8), and fibrosarcoma and melanoma (9), but have not been evaluated for PDA.
An increasing number of studies have assessed pancreatic cancer treatment response with T1-weighted dynamic gadolinium contrast-enhanced magnetic resonance imaging (CE-MRI). CE-MRI studies have shown that antiangiogenic treatment decreases perfusion parameters before detection of a change in tumor size, in a rat model (10) and in human patients (11). Unfortunately, the addition of antiangiogenic therapy to PDA treatment have not shown benefit in clinical trials (12,13) and are no longer commonly utilized. A recent CE-MRI study in human patients treated with currently used regimens (FOLFIRINOX, gemcitabine plus protein-bound paclitaxel, or gemcitabine plus cisplatin) found significantly increased perfusion in PDA tumors responding to therapy. To account for the inherent variability in CE-MRI measurements, an external perfusion phantom was used for calibration (14).
Effective PDA treatment results in therapy-related increased fibrosis and reduced tumor cell volume (15,16). Fibrosis may manifest on CE-MRI as increased contrast uptake on delayed phase T1-weighted images, a property that has been demonstrated in other fibrotic tissues related to cardiac (17) and liver (18) imaging. We hypothesize that treatment-related fibrosis in pancreatic cancer should result in an increased contrast uptake on delayed phase MRI. In patients with tumor response, fibrosis may decrease unenhanced T1 signal intensity (19), while injury response and granulation tissue or remodeled ingrowth of pancreatic acinar tissue may be expected to increase T1 signal intensity (8,9). In the absence of treatment response, we expect little change in the tissue histology or MRI features. To evaluate changes of PDA response to treatment on CE-MRI and to test these hypotheses, we developed a quantitative metric reflecting both T1 signal intensity and contrast uptake that can be computed from standardized clinical MRI exams; we applied this metric in a retrospective study in groups of PDA patients that did, or did not, respond to treatment.
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). We obtained prior to initiating research an Institutional Review Board Approval Protocol Number: 1704366654 and further obtained a waiver of personal health information authorization [45 CFR 164.512(i)(2) (ii)] as the use or disclosure of protected health information involves no more than minimal risk to the individuals and the research could not practicably be conducted without the waiver.
Patients diagnosed with pancreatic cancer at the University of Arizona Cancer Center from January 2012 to June 2016 were consecutively included. Inclusion criteria were: (I) at least 18 years of age; (II) borderline unresectable, or locally advanced unresectable, or metastatic PDA; (III) MRI examination before and after treatment with chemotherapy and/or radiation; (IV) measurable disease on MRI; and (V) CA 19-9 serum tumor biomarker both expressed and measured at time of MRIs. Patients were included regardless of prior treatment. All patients had fine-needle aspiration or core biopsy tissue sampling by endoscopic ultrasound with histologically confirmed PDA. The stage of cancer was determined by imaging (20).
Further criteria were used to classify two patient groups as (I) responder or (II) non-responder. Patients in the responder group had (I) at least 30% decrease in maximum length after treatment [partial response per RECIST 1.1 (2)] or (II) CA 19-9 decrease by at least 50% with treatment (21,22) without regard to tumor size in cases without metastatic disease. CA 19-9 was not used to assess treatment response in the setting of metastases since the decrease in CA 19-9 may be potentially be due to decrease in metastases rather than the pancreatic mass. The remaining patients comprised the non-responder group. Out of the initially identified 264 patients, these criteria produced a total of 35 patients, with 24 patients in the responder group and 11 patients in the non-responder group (Figure 1, Table 1). Among the 24 responders, 17 were assessed to be responders by size changes, 4 by CA 19-9 changes, and 3 by both size and CA 19-9 changes.
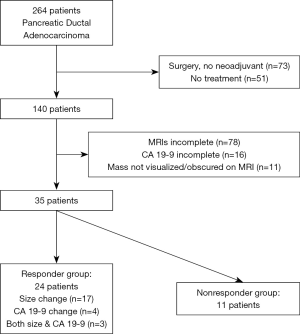
Table 1
| Characteristics | Non-responder (n=11) | Responder (n=24) | P value |
|---|---|---|---|
| Age (years) | 68.9±8.2 | 65.1±10.6 | 0.257 |
| No. of females | 5/11 (45.5%) | 11/24 (45.8%) | 1.000 |
| Size (cm) | 3.17±1.27 | 3.49±0.91 | 0.471 |
| CA 19-9 (U/mL) | 2,901.9±5,862.1 | 2,898.6±5,612.1 | 1.000 |
| Treatment regimen | 0.115 | ||
| Gemcitabine ± protein-bound paclitaxel | 2/11 (18.2%) | 6/24 (25.0%) | |
| FOLFIRINOX ± pegph20 | 7/11 (63.6%) | 15/24 (62.5%) | |
| Other§ | 2/11 (18.2%) | 3/24 (12.5%) | |
| Pre-treatment MRI (days before start of treatment)† | 22 [7–38] | 22 [10.5–31.5] | 0.422 |
| Post-treatment MRI (days after start of treatment)† | 85 [53–105] | 75.5 [52.5–82.5] | 0.147 |
| Disease stage | |||
| Borderline | 6/11 (54.5%) | 11/24 (45.8%) | |
| Locally advanced | – | 3/24 (12.5%) | |
| Metastatic | 5/11 (45.5%) | 10/24 (41.7%) | |
| Borderline tumors resected | 3/6 (50.0%) | 7/11 (63.6%) |
†, Reported as median [inter-quartile range]. §, Other treatment regimens include HAPa vaccine (1 responder), capecitabine (1 each of responder and non-responder), and radiation therapy (1 each of responder and non-responder). CA 19-9, cancer antigen 19-9; MRI, magnetic resonance imaging.
All patients received chemotherapy and/or radiation, with the majority (22/35 patients) receiving FOLFIRINOX (Table 1). All forms of treatment relied on DNA synthesis inhibition (12), unlike prior MRI studies which employed antiangiogenic medications (10,11). Patients received MRIs before and after treatment as part of standard protocol. CA 19-9 was measured at the time of each MRI.
Imaging protocol
All studies were performed on 1.5 or 3.0 T MRI systems (Siemens Medical Solutions, Magnetom Aera/Skyra, Erlangen, Germany). Patients were imaged in the supine position with a phased array torso coil. Patient received 0.05 mmol/kg gadobenate dimeglumine (MultiHance; Bracco Diagnostics, Milan, Italy) intravenous contrast administered by power injection at a rate of 2 mL/s, followed by 20 mL of saline bolus flush at 2 mL/s. Axial three-dimensional T1-weighted gradient echo non-contrast and post-contrast images were acquired through the abdomen at the arterial, venous (45 s) and delayed phases (180 s), each acquired during a breath-hold. Arterial phase images were acquired by automated bolus-triggered technique (Automated Bolus Liver Exam, Liver Lab, Siemens Medical Systems). Technical parameters for the 1.5 T included: TR 4.47 ms, TE 2.2 ms, flip angle 10°, slice thickness 3.0 mm, matrix size 288×216. Technical parameters for the 3.0 T were: TR 3.13 ms, TE 1.13 ms, flip angle 9°, slice thickness 3.0 mm, matrix size 288×216. For both 1.5 and 3 T, technical parameters were: two-dimensional generalized autocalibrating partially parallel acquisitions parallel imaging (2D-GRAPPA) with acceleration factor of 2×2, partial sampling 78% phase field-of-view and 80% phase resolution, and total scanner room utilization times of 25 minutes for abdomen and 45 minutes abdomen and pelvis.
Image analysis
Size measurements and region of interest (ROI) placement
The long axis of the pancreatic masses was measured on the imaging phase where the mass was best visualized (non-contrast or arterial) on axial images (JL with 7 years of abdominal imaging experience). The change of the tumor length with treatment was used to determine partial response with size decrease of at least 30% [RECIST 1.1 guidelines (2)].
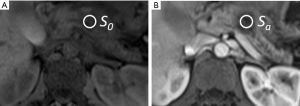
Circular ROIs were positioned on the pancreatic mass using the PACS system (Figure 2) to obtain average signal intensities of the pancreatic mass on T1-weighted (I) non-contrast image (S0) and post-contrast (II) arterial (Sa), (III) venous (Sv), and (IV) delayed (Sd) phase images. These four measurements were taken for the pre-treatment and post-treatment MRIs of each patient on a single axial slice for each contrast phase. The ROIs were selected to include the majority of the central portion of the pancreatic mass, excluding the sometimes heterogeneous periphery. In large masses that spanned multiple imaging slices, care was taken to select the same portion of the mass in the pre-treatment and post-treatment MRIs. Signal intensity measurements were made by an abdominal imaging fellow (TM with 5 years of abdominal imaging experience), who was blinded to the identity of the pre and post-treatment conditions and whether patients were in the responder or non-responder groups; in order to keep this reader blinded to the efficacy of treatment, he did not make tumor size measurements or view the CA 19-9 data.
Quantitative metric
We sought to develop an objective measure of contrast media uptake in pancreatic masses on conventional pre- and post-contrast T1-weighted images. The contrast media uptake cannot be separated from lesion T1 on conventional MRIs, which lack quantitative T1 mapping. Using first principles for the signal provided by the gradient echo MRI sequence (23), the relationship of T1 to contrast media (24), and our MRI scan parameters, we theoretically derived a quantitative metric (T1C) which is the product of longitudinal relaxation time T10 and contrast media concentration at the post-contrast phase CMi (Appendix 1):
For each patient, MRI scan, and post-contrast phase, signal-ratio (SR) was computed as:
Where S is the signal intensity of the pancreatic mass on the T1-weighted image, 0 is the pre-contrast phase, and i is the post-contrast phase (arterial a, portal venous v, and delayed d). T1C is then calculated by:
Where b is a fit parameter accounting for the two scanner magnetic field strengths used in this study and r1(B0) is the field-strength dependent relaxivity of the contrast media in plasma (Appendix 1).
Statistical methods
Treatment regimen and biological sex were numerically coded. Since the number of patients treated with capecitabine, HAPa vaccine and radiation therapy were low (2 or 1), they were grouped together. Continuous variables were assessed for normal distribution (Shapiro-Wilk’s test) and appropriate summary statistics were obtained. Baseline characteristics of the patient were analyzed to determine if they differed between responders and non-responders. For continuous variables satisfying the normality assumption, independent t-test with Satterthwaite correction was used for analysis. For variables reported as a proportion, Fisher’s exact test was used to determine if they differed between responders and non-responders. For ordinal variables, Wilcoxon-Mann-Whitney test was used for analysis. Generalized linear models (repeated measures analysis of variance) was used to determine if T1C at multiple post-contrast phases differed between pre-treatment and post-treatment scans for responders and non-responders. Above analyses were performed using statistical software (SAS®9.4, SAS Institute Inc., Cary, NC, USA). For each patient group and phase, means, standard deviations (SDs), and paired t-tests were computed using Matlab (Mathworks, Natick, MA, USA). Effects associated with P<0.05 were considered statistically significant.
Results
The baseline characteristics are summarized in Table 1. None of the baseline characteristics significantly differed between responders and non-responders (P>0.115). With treatment, the CA 19-9 decreased by 35%±54% in the responder group and increased by 849%±2,339% in the non-responder group. Pancreatic mass size decreased by 35%±15% in the responder group and increased by 10%±37% in the non-responder group.
Repeated measures analysis of variance indicated that the pre-treatment vs. post-treatment T1C significantly differed between responders and non-responders (P=0.044, between-subject effects), with the T1C being larger in the post-treatment vs. pre-treatment MRI for responders compared to non-responders in all three post-contrast phases. Multivariate tests of within-subject effects analysis showed a significant change in T1C between post-contrast phases (P<0.0001), that the effect of post-contrast phase on T1C was not dependent on whether it was pre-treatment or post-treatment (P=0.985), that the effect of post-contrast phase on T1C was not dependent on whether the subjects were responders or non-responders (P=0.247), and the effect of post-contrast phase on T1C was not dependent on the four combinations of pre-/post-treatment and responder/non-responder (P=0.947). Figure 3 shows the least-squares means of T1C along with the standard error at each post-contrast phase for responders and non-responders.

T1C in responders
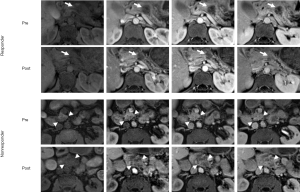
Consistent with our hypothesis that pancreatic cancer treatment response can be observed in MRI through altered contrast uptake, we found significant increases in T1Ca, T1Cv and T1Cd after treatment (P=7.57e–5, 3.25e–4 and 2.75e–4, respectively, Figure 3, Table 2). These findings are illustrated in an example subject in the top two rows of Figure 4.
Table 2
| Patient group | Quantitative metric | Pre-treatment (mean ± SD) | Post-treatment (mean ± SD) |
|---|---|---|---|
| Responder | T1Ca | 0.10±0.09 | 0.21±0.10 |
| T1Cv | 0.15±0.11 | 0.26±0.12 | |
| T1Cd | 0.24±0.13 | 0.36±0.15 | |
| Non-responder | T1Ca | 0.10±0.09 | 0.10±0.06 |
| T1Cv | 0.17±0.14 | 0.14±0.07 | |
| T1Cd | 0.22±0.13 | 0.21±0.13 |
SD, standard deviation.
T1C in non-responders
We hypothesized that treatment failure should result in no change in contrast uptake. We found that T1C did not change significantly in any of the post-contrast phases in the non-responder group (P>0.582, Table 2). The lack of increase in contrast media uptake is illustrated in an example subject in the bottom two rows of Figure 4.
Discussion
We derived a quantitative metric T1C, which is linearly related to T1 and contrast media uptake and can be applied to standardized clinical MRI scans. We found that T1C was significantly increased after treatment in all post-contrast phases in the responder group but not the non-responder group. In responders, the results imply three possible scenarios could have contributed to the increase in T1C in post-treatment MRI: an increase in lesion T1, an increase in contrast uptake between pre-contrast and post-contrast phases, or a combination of the above.
Although the contrast uptake cannot be isolated from lesion T1 in our study, post-contrast images clearly show increased contrast uptake after treatment in the responder group (Figure 4). This is in agreement with recent CE-MRI (14) and perfusion CT (25) studies demonstrating increased blood flow to PDA tumors with successful treatment. These two independent prior reports suggested a similar etiology for the increased perfusion after treatment. They proposed that PDA is normally hypoperfused due to compression of tumor vessels by tumor sclerosis or extracellular matrix scaffold. Effective treatment loosens the scaffold and reduces interstitial pressure, increasing perfusion (14,25). This may correspond to the increased T1Ca and T1Cv in the responder group of our study. Treatment-related inflammation can increase blood flow through angiogenesis and would also produce these results (26,27). Tissue biopsies may be another approach to test these possible correlations.
We found that T1Cd significantly increased with PDA response to treatment, indicating an increase in delayed phase contrast uptake. Histologically, treatment response manifests as the overgrowth and/or replacement of viable tumor with fibrosis (15,16) over time. Fibrosis is associated with an increase in contrast uptake in delayed phase imaging in cardiac (17) and liver (28) MRI, and may be a factor in the T1Cd effect measured in PDA that have responded to treatment.
Decreased T1 can be seen with release of proteins and/or metals from cell and tissue destruction in mouse models of fibrosarcoma and melanoma (9) and with intra-acinar necrosis in colorectal liver metastases (29). The increase in T1C we found in our study suggests that positive treatment response produced (I) an increased lesion T1 or (II) an increased contrast uptake to a greater extent than a decreased T1; the latter of these possibilities would be concordant with the cited studies. Also, increased fibrosis has been shown to increase T1 in the heart (19), and the known development of fibrosis with successful treatment response in PDA (15,16) may well have similar signal characteristics. It is possible that PDA responding to treatment may develop various factors that induce change of T1. These changes may be in relation to fibroinflammatory infiltrates that replace tumor, or as a result of cellular apoptosis or necrosis. Future studies, including T1-mapping, may be helpful for further characterization with greater accuracy and sensitivity to T1 properties of PDA, beyond what was possible with standard T1-weighted 3D GRE, as acquired in our study.
Our study has several limitations. Although we started with 264 patients, the final study population was small (n=35) due to the strict inclusion criteria (Figure 1). Patients with resectable disease did not routinely receive neoadjuvant therapy at our institution, leading to exclusion of 73 patients who went straight to surgery. A further 51 patients elected not to have any treatment. Out of the remaining patients, 78 had incomplete MRIs (missing pre-treatment or post-treatment MRI or insufficient images), 16 had incomplete CA 19-9 measurements, and 11 had masses there were not visible on MRI or were obscured by biliary stents. The retrospective design of this pilot study introduced variability in the timing and type of chemotherapy or radiation and also the MRI timing, relative to treatments. A future prospectively designed study may yield a higher inclusion rate and introduce less opportunities for hidden bias. A larger sample size may produce improved statistical significance. Measures of inter- or intra-observer variability were not performed in this pilot study, and future work including this may be helpful.
We chose tumor size and CA 19-9 changes as the reference standards for treatment response here since they are the most commonly used parameters in clinical practice. Histologic evidence of treatment response was not available in the majority of our patients since they did not undergo surgical resection, and use of progression-free survival or overall survival is complicated by subsequent treatments (i.e., surgical resection, chemotherapy, radiation) received by many patients in our study. Tumor size and CA 19-9 changes are imperfect reference standards and do not always agree, as evidenced by the 21/24 responders here that did not have concordant qualifying tumor size and CA 19-9 changes. An independent marker such as T1C would be especially useful when tumor size and CA 19-9 do not agree, and when tumor size changes are equivocal and CA 19-9 is not measurable. Our preliminary results warrant further testing as a prospective cancer trial which allows for incorporation of additional reference standards.
Several variables affect the T1C values, some of which are present in this study, and others of which would arise with use of different scan protocols. Variations in magnetic field homogeneity are present in every MRI exam. These are included in Eq. [4] in the instrument scaling constant k. Since magnetic field inhomogeneity is relatively constant within a scan session, k is the same in both the numerator and denominator of the ratio in Eq. [7]. This allows it to cancel out and makes T1C relatively insensitive to magnetic field inhomogeneity. Differences in patient weight and physiology may be present in studies of different patients, or in the same patient over time. This could affect the amount of contrast reaching a pancreatic mass, with larger patients or slower circulation resulting in decreased contrast accumulation and decrease in T1C. These effects could contribute to variability in the results; however, our results were statistically significant despite the presence of these potential sources of variability. Although not present in our study, use of different scan protocols would also introduce variability if not accounted for. Specifically, b depends on TR and flip angle θ per Eq. [5]. If different parameters from ours were used or the same protocol is not used throughout a study, b will need to be adjusted by performing a power-law fit. Using typical TR values of 2–5 ms (30-32), b ranges from –0.80966 to –6.3308, when flip angle θ is 10°. Typical flip angle θ values of 9°–12° (30-32) produces b-values of –0.6100697 to –0.7342758, when TR is 4.47 ms. Use of a different contrast dose or infusion rate would also introduce variability similar to differences in patient physiology above. For a given contrast media, an increase in contrast dose would increase T1C in a linear manner per Eq. [13]. When different contrast media are used between scans, this is accounted by r1(B0), which is the field-strength dependent relaxivity of the contrast media in plasma, as per Eq. [14]. The metric reflects the concentration of the contrast media and hence all factors affecting the concentration of contrast media, such as the infusion speed will also be represented in the metric. Ideally, scan protocols would be uniform within a study population, reducing the sources of error in T1C discussed above. Comparison of T1C across different studies with different scan protocols are more complex and may require adjustments to the model.
Our T1C method may be applied to conventional CE-MRI examinations. Accurate T1-mapping with adequate spatial and temporal resolution would be preferable, providing more accurate and potentially sensitive indicators of intrinsic T1 tumor properties and changes related to treatment, pre- and post-contrast enhancement. However, T1-mapping for this application remains technically challenging, although this may be possible in the future. In addition, we did not find T1C values served to prognosticate a responder from a non-responder in the pre-treatment assessment of tumors. Previously, it has been shown that MRI arterial enhancement correlated with PDA grade on histology (33). It may be that we would expect differences in tumor response depending on grade and that we may have found differences in the pre-treatment MRI. This may require a different study design and a larger sample size for delineating possible differences that may prognosticate tumor responsiveness. The data in this study was acquired as part of clinical exams which included diffusion-weighted imaging and apparent diffusion coefficient (ADC) maps. The changes in ADC with treatment were explored but found to be unreliable due to motion artifact. While ADC may be an additional path of investigation, it would require a different methodology for signal acquisition and/or processing.
This preliminary work has potential application and clinical impact for improving assessment of PDA treatment response. PDA has poorly defined margins and is notoriously difficult to measure size accurately and precisely (6). Also, size measurements cannot distinguish between residual tumor and fibroinflammatory tissue after treatment (3-5). A quantitative metric reflecting T1 and contrast uptake would be a useful adjunct to size measurements from imaging. The T1C methodology used in our study may be applied to most standard clinical CE-MRIs. Having this additional option is especially important when size changes by imaging are equivocal and CA 19-9 is not available. Given the significant findings in this retrospective study using standard abdominal MRIs, further validation of T1C is warranted in larger prospective clinical trials and may be useful in radiomics applications.
Conclusions
We derived a quantitative T1C metric that is related to T1 and contrast uptake that can be used with standard clinical CE-MRI. This metric increased in pancreatic cancer responders but not non-responders, possibly providing an alternative to size measurements in assessing treatment response.
Acknowledgments
Funding: This work was supported by 2P30 CA023074 supplement of the Cancer Center Support Grant from the NCI/NIH to the University of Arizona Cancer Center. The contents are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH/NCI.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apc-20-25
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-20-25). Dr. SV reports grants from NIH/NCI during the conduct of the study. HB serves as an unpaid Section Editor of Annals of Pancreatic Cancer from October 2019 to September 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). We obtained prior to initiating research an Institutional Review Board Approval Protocol Number: 1704366654 and further obtained a waiver of personal health information authorization [45 CFR 164.512(i)(2) (ii)] as the use or disclosure of protected health information involves no more than minimal risk to the individuals and the research could not practicably be conducted without the waiver.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yeo TP. Demographics, epidemiology, and inheritance of pancreatic ductal adenocarcinoma. Semin Oncol 2015;42:8-18. [Crossref] [PubMed]
- Minocha J, Lewandowski RJ. Assessing imaging response to therapy. Radiol Clin North Am 2015;53:1077-88. [Crossref] [PubMed]
- Cassinotto C, Mouries A, Lafourcade JP, et al. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 2014;273:108-16. [Crossref] [PubMed]
- Dudeja V, Greeno EW, Walker SP, et al. Neoadjuvant chemoradiotherapy for locally advanced pancreas cancer rarely leads to radiological evidence of tumour regression. HPB (Oxford) 2013;15:661-7. [Crossref] [PubMed]
- Wagner M, Antunes C, Pietrasz D, et al. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 2017;27:3104-16. [Crossref] [PubMed]
- Arvold ND, Niemierko A, Mamon HJ, et al. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 2011;80:1383-90. [Crossref] [PubMed]
- Mattes MD, Cardinal JS, Jacobson GM. Delayed radiation-induced inflammation accompanying a marked carbohydrate antigen 19-9 elevation in a patient with resected pancreatic cancer. Radiat Oncol J 2016;34:156-9. [Crossref] [PubMed]
- Jamin Y, Tucker ER, Poon E, et al. Evaluation of clinically translatable MR imaging biomarkers of therapeutic response in the TH-MYCN transgenic mouse model of neuroblastoma. Radiology 2013;266:130-40. [Crossref] [PubMed]
- Weidensteiner C, Allegrini PR, Sticker-Jantscheff M, et al. Tumour T1 changes in vivo are highly predictive of response to chemotherapy and reflect the number of viable tumour cells--a preclinical MR study in mice. BMC Cancer 2014;14:88. [Crossref] [PubMed]
- Raatschen HJ, Fischer S, Zsivcsec B, et al. Non-invasive quantification of anti-angiogenic therapy by contrast-enhanced MRI in experimental pancreatic cancer. Acta Radiol 2014;55:131-9. [Crossref] [PubMed]
- Akisik MF, Sandrasegaran K, Bu G, et al. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology 2010;256:441-9. [Crossref] [PubMed]
- Caparello C, Meijer LL, Garajova I, et al. FOLFIRINOX and translational studies: Towards personalized therapy in pancreatic cancer. World J Gastroenterol 2016;22:6987-7005. [Crossref] [PubMed]
- Saif MW. Pancreatic cancer: Sorafenib: no effect on efficacy of chemotherapy in pancreatic cancer. Nat Rev Gastroenterol Hepatol 2014;11:8-9. [Crossref] [PubMed]
- Kim H, Morgan DE, Schexnailder P, et al. Accurate therapeutic response assessment of pancreatic ductal adenocarcinoma using quantitative dynamic contrast-enhanced magnetic resonance imaging with a point-of-care perfusion phantom: a pilot study. Invest Radiol 2019;54:16-22. [Crossref] [PubMed]
- Hartman DJ, Krasinskas AM. Assessing treatment effect in pancreatic cancer. Arch Pathol Lab Med 2012;136:100-9. [Crossref] [PubMed]
- Pai RK, Pai RK. Pathologic assessment of gastrointestinal tract and pancreatic carcinoma after neoadjuvant therapy. Mod Pathol 2018;31:4-23. [Crossref] [PubMed]
- Vogel-Claussen J, Rochitte CE, Wu KC, et al. Delayed enhancement MR imaging: utility in myocardial assessment. Radiographics 2006;26:795-810. [Crossref] [PubMed]
- Martin DR, Lauenstein T, Kalb B, et al. Liver MRI and histological correlates in chronic liver disease on multiphase gadolinium-enhanced 3D gradient echo imaging. J Magn Reson Imaging 2012;36:422-9. [Crossref] [PubMed]
- Okur A, Kantarci M, Kizrak Y, et al. Quantitative evaluation of ischemic myocardial scar tissue by unenhanced T1 mapping using 3.0 Tesla MR scanner. Diagn Interv Radiol 2014;20:407-13. [Crossref] [PubMed]
- Tamm EP, Silverman PM, Charnsangavej C, et al. Diagnosis, staging, and surveillance of pancreatic cancer. AJR Am J Roentgenol 2003;180:1311-23. [Crossref] [PubMed]
- Ishii H, Okada S, Sato T, et al. CA 19-9 in evaluating the response to chemotherapy in advanced pancreatic cancer. Hepatogastroenterology 1997;44:279-83. [PubMed]
- Ko AH, Hwang J, Venook AP, et al. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 2005;93:195-9. [Crossref] [PubMed]
- Bernstein MA, King KF, Zhou XJ. Handbook of MRI Pulse Sequences. Burlington: Elsevier Inc.; 2004.
- Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005;40:715-24. [Crossref] [PubMed]
- Hamdy A, Ichikawa Y, Toyomasu Y, et al. Perfusion CT to assess response to neoadjuvant chemotherapy and radiation therapy in pancreatic ductal adenocarcinoma: initial experience. Radiology 2019;292:628-35. [Crossref] [PubMed]
- Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis 2007;10:149-66. [Crossref] [PubMed]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev 2007;65:S140-6. [Crossref] [PubMed]
- Petitclerc L, Sebastiani G, Gilbert G, et al. Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging 2017;45:1276-95. [Crossref] [PubMed]
- Milot L, Guindi M, Gallinger S, et al. MR imaging correlates of intratumoral tissue types within colorectal liver metastases: a high-spatial-resolution fresh ex vivo radiologic-pathologic correlation study. Radiology 2010;254:747-54. [Crossref] [PubMed]
- Li XH, Zhu J, Zhang XM, et al. Abdominal MRI at 3.0 T: LAVA-Flex compared with conventional fat suppression T1-weighted images. J Magn Reson Imaging 2014;40:58-66. [Crossref] [PubMed]
- Donato H, França M, Candelária I, et al. Liver MRI: from basic protocol to advanced techniques. Eur J Radiol 2017;93:30-9. [Crossref] [PubMed]
- Chang KJ, Kamel IR, Macura KJ, et al. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics 2008;28:1983-98. [Crossref] [PubMed]
- Lauenstein TC, Martin DR, Sarmiento JM, et al. Pancreatic adenocarcinoma tumor grade determination using contrast-enhanced magnetic resonance imaging. Pancreas 2010;39:71-5. [Crossref] [PubMed]
Cite this article as: Liau J, Vedantham S, Babiker HM, McGlothin T, Martin DR. Quantitative metric for assessment of pancreatic ductal adenocarcinoma treatment response in T1-weighted gadolinium-enhanced magnetic resonance imaging. Ann Pancreat Cancer 2020;3:15.


