
A national database analysis of acinar cell carcinoma of the pancreas, a histologically, epidemiologically, and biologically distinct entity increasing in incidence
Introduction
Pancreatic acinar cell carcinoma (PACC) is a rare form of exocrine pancreatic cancer which represents only 1–2% of pancreatic cancer diagnoses (1-3). It is more common in males with a lower mean age at diagnosis compared to pancreatic ductal adenocarcinoma (PDAC) (1). Histologically it can appear as acinar cells or may be comprised of poorly differentiated cells making the morphologic diagnosis difficult. PACCs produce specific enzymes including proteases, lipases, and amylases, which can aid in diagnosis via immunohistochemical staining (4). PACC is assumed to have a better prognosis than PDAC, but is still poor with 5 year survival of only 10% (1). The preferred treatment for localized PACC tumors has been surgical resection not unlike PDAC. Resection of these cancers has been associated with increased long-term survival (4). Metastatic disease is typically not curable and is treated with systemic chemotherapy.
Due to the low incidence of disease, no studies have been able to identify the appropriate treatment regimen for patients with PACC in a randomized fashion. It is unclear what the role of chemotherapeutic agents is and what the sequence of treatment should be. Furthermore, very few studies have used large population databases to compare the characteristics, outcomes and incidence of PACC to PDAC (5,6). Reports of neoadjuvant chemotherapy (NAC) for patients with PACC are limited to case reports and small-case series, and there remains minimal data comparing the survival of patients with PACC versus PDAC treated with upfront resection versus neoadjuvant therapy (7-10). A better understanding of how patients respond to therapies is needed.
In this study, characteristics of both PACC and PDAC were compared using data from the NCDB. We evaluated the differences in stage specific survival for all stages of disease and compared them between PACC and PDAC. We then compared survival for resected PACC patients compared to resected PDAC. Additionally, since an analysis of the incidence of PACC has not been assessed in recent years, we used the SEER database to analyze the change in incidence of PACC from the years of 2005–2015. This incidence trend of PACC was compared to that of PDAC. To our knowledge, no previous studies have used two large national databases to evaluate the relationship of these two entities.
Methods
SEER data analysis
The Surveillance Epidemiology and End Results (SEER) is a population-based source of data from 9 state-based registries in the United States (US) (11). We compared incident cases of pancreatic acinar cell carcinoma and ductal adenocarcinoma for the time period available in our NCDB Participant User File (PUF – 2005-2015). ICD-O-3 topographical codes (C25.0-25.9) and histologic (8140 for PDAC and 8550 for PACC) codes were utilized to identify patients. Incidence was determined per 100,000 population adjusted to the standard population of the US in the year 2000. Percent change on an annual basis was calculated using the linear least-squares method and subsequently tested for deviation from zero using a t-distribution regression. Trends were assessed for significance using Cochran-Armitage trend analysis.
NCDB data analysis
Histopathologic, demographic, and outcome comparisons were performed as a cohort study of the NCDB participant user file (PUF) of patients presenting for curative-intent surgery of pancreatic acinar cell carcinoma and ductal adenocarcinoma during the time period outlined above. The NCDB contains records from over 30 million cancer cases treated at more than 1500 Commission on Cancer (CoC) approved hospitals in the US and is expected to capture roughly 70% of newly diagnosed cases of cancer in the US (12).
Patients with acinar cell carcinoma and ductal adenocarcinoma were sought using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography (25.0–25.9) and histology codes (8140 for PDAC, 8550 for PACC). Curative-intent surgery was defined as surgery of primary site codes 20–90. Patients with surgical codes 0 (no surgery) and 99 (Unknown) were considered to have had no curative intent surgery. Patients not treated at the reporting hospital were excluded. TNM staging is recorded in the NCDB using American Joint Commission on Cancer (AJCC) 6th/7th edition staging classification dependent on year of diagnosis, and the ‘analytic stage group’ variable was used for staging. This variable is based on pathologic staging where available and clinical staging where pathologic staging information is missing. Patients with missing follow-up or final pathologic staging data were excluded as were those who developed cancer at more than one site.
Statistical analysis
Normally distributed continuous data were treated with mean/standard deviation (SD) and significance was tested with a student’s t-test (two-tailed). Continuous data with non-normal distributionwere expressed as median and inter-quartile range (IQR) with significance testing performed using the Mann-Whitney U-test. Categorical variables with uniform distribution were assessed for significance with Pearson’s chi-square tests while categorical variables with non-uniform distribution were tested with the Fisher’s exact test. Missing data were tracked with indicator variables as shown in the tables.
The Kaplan-Meier method was used for survival estimation with the definition of survival being time from diagnosis to death or censor. Significance testing comparison of survival curves was performed with the log-rank test. Overall survival is reported due to the fact that the NCDB does not provide data on recurrence or cause of death. To estimate independent impact of covariates on survival, the method of Cox proportional hazards was developed in resected patients which included tumor subtype, clinical stage, tumor grade, node status, margins, and receipt of radiation and/or chemotherapy. Statistical significance was set at a P<0.05. Analysis of data was performed using R version 3.5.2 “Eggshell Igloo” (R Foundation for Statistical Computing – Vienna, Austria www.r-project.org). The Mayo Clinic Institutional Review Board has deemed this study exempt from review. The study was conducted in compliance with the Helsinki Declaration (revision from 2013).
Results
We identified 137,962 patients with PDAC and 589 with PACC in the NCDB. There were several features which were significantly different between the two tumor types which is detailed in Table 1. Patients with PACC were more likely to be male (72.8% vs. 51.7%) and were younger at the time of diagnosis (64.0 vs. 68.0 years old). Patients with PACC had significantly larger tumors (5.4 vs. 4.2 cm) at the time of diagnosis. Patients with PACC were more likely to have nodal involvement (49.3% vs. 28.2%) and were less often located in the head of the pancreas (44.5% vs. 51.7%). A minority of patients treated with systemic chemotherapy were sequenced in neoadjuvant fashion (2.8% PDAC and 4.4% PACC). Patients with PACC more often underwent curative-intent surgery (43.8% vs. 16.4%) and were less likely to have positive resection margins (20.4% vs. 24.2%). There was no statistically significant difference between PACC and PDAC amongst the entire cohort of patients diagnosed during the study period in regards to number of patients receiving chemotherapy or radiation therapy, race, Charlson Deyo score, or year of diagnosis. The interval survival for patients with PACC was better at 1, 2, and 3 years compared to PDAC.
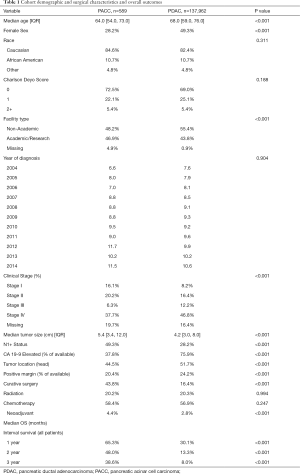
Full table
Cox proportional hazard model for resected patients revealed several statistically significant predictors of mortality hazard and can be seen in Table 2. Among resected patients, predictors of mortality included stage three and four tumors; stage 3 (HR: 1.26; 1.16–1.36) and stage 4 (HR: 1.85; 1.70–2.01). Additional predictors included high tumor grade (HR: 1.32, 1.27–1.36), positive resection margin (HR: 1.62, 1.56–1.68), omission of radiation (HR: 1.12; 1.08–1.16). After adjustment for stage, grade, nodal status, margins, chemotherapy and radiation, PDAC histology remained the strongest independent predictor of mortality (HR 2.33, 95% CI: 1.95–2.78).
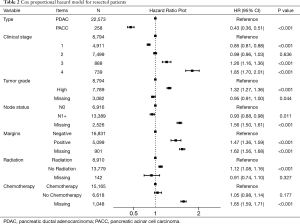
Full table
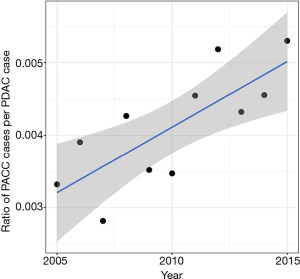
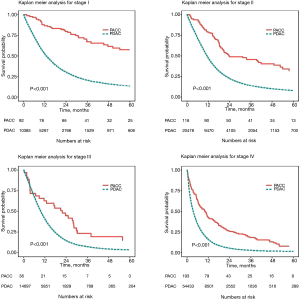
Incidence of PACC increased by 73% over the twelve year study period, while the incidence of PDAC increased by only 22%. This difference was statistically significant with P<0.01. The ratio of incidence change between PACC vs. PDAC was also significant at 59% increase over the study period (Figure 1). Stage-specific survival was found to be significantly better for all stages of PACC when compared to PDAC (Figure 2). Stage I disease showed the greatest difference in survival probability. Kaplan-Meier analysis for all stages combined and in resected patients also demonstrated improved survival for patients with PACC compared to PDAC and can be seen in Figure 3. Kaplan-Meier analysis was performed over a 60-month period and survival was found to be higher for patients with PACC for the duration of this period of time in all analyses performed.

Discussion
In the present study, we investigated the increased incidence of PACC and identified improved overall survival for patients with PACC as compared to PDAC. Despite PACC presentation with larger tumor size, there is a lower likelihood of lymph node involvement and patients with PACC are less likely to have positive margins at the time of surgical resection. These findings, along with the improved overall survival at all stages, suggests that PACC is a less aggressive malignancy than PDAC. The 73% increase in incidence over a 12 year study period is of significant interest. The study demonstrated that the incidence is increasing at a much faster rate than PDAC. The reason for the increasing incidence is unknown and is a subject for further investigation which should be followed with interval studies. Possible explanations for this increased incidence is improvements in the ability to identify this histologic subtype, increased incidental identification during abdominal imaging for other purposes, or due to genetic factors.
Due to its rarity, there is a lack of information in the current literature describing epidemiologic or modifiable risk factors for PACC. However, elucidating genetic risk factors and associations may aid investigators in identification of pathways involved in the development of PACC and, subsequently, development of specific targeted therapies for the disease. Studies investigating the molecular characterization of PACC have identified a number of associated genetic mutations (4,13,14). Genes which have been identified as mutant in select tumors include TP53, BRAF, SMAD4, CDK2NA, MMR/MSI, RB1, APC, JAK1, MEN1, BRCA1, BRCA2, ATM, PALB2, and MSH2, several of which are targetable action genes (4,13,14). One particularly interesting gene of those identified is MMR/MSI which has therapeutic implications. Pembrolizumab is a PD-L1 inhibitor that is FDA approved as of October 2020 for use in all MMR/MSI unresectable solid tumors which have progressed following treatment with no alternative viable options (15). The rate of microsatellite instability in PACC tumors has been reported to range from 8–15%, which is much higher than that reported in PDAC (1–2%) (2,4,16). There is no data recommending a change in therapy based on tumor instability, however, given the availability and potential benefit of these targeting therapies, it would be prudent to obtain MSI information in cases of PACC as it may broaden systemic therapy options. Another intriguing genetic finding is that KRAS mutations are found less frequently PACC when compared to PDAC. This may provide a molecular basis for the improved survival as patients with KRAS wildtype PDAC have a better prognosis than those with KRAS mutations (16).
Since tumor biology portends a significant variation in response to treatment modalities, it will become increasingly important to determine histologic subtype when formulating a treatment plan. Determining histologic subtypes can be difficult. The presentation of PACC can be non-specific although there are some distinct differences unique to that of ductal carcinoma. For example, the finding of elevated lipids is seen in 50% of patients, with up to 10% of patients having lipase hypersecretion syndrome, which presents as subcutaneous fat necrosis, polyarthralgia, occasional eosinophilia and nonbacterial thrombotic endocarditis (1). These are often absent and may create difficulties in the diagnosis of PACC (2).
Despite being clinically absent in many patients these unique features can aid in diagnosis (1,16). Serum lipase is a tumor marker for PACC and should be checked (17). Distinct imaging findings also can help to differentiate PACC from PDAC, although they are less helpful at differentiating from other tumors of the pancreas. Computed tomography (CT) is considered the standard diagnostic modality for PACC (18). On CT, PACC has central hypodensity, a well-defined enhancing capsule and internal calcifications (19,20). In order to achieve the correct diagnosis, pathology, tumor presentation and imaging findings should be carefully reviewed. Once histology is identified, the treatment can be tailored to tumor subtype and response to treatment can be studied for application to future patient treatment planning.
In conclusion, it is important to recognize that, although it has a more favorable prognosis than its ductal counterpart, PACC is an aggressive cancer that requires aggressive treatment. When surgical resection is pursued an R0 resection should be the goal (18). The role of NAC in increasing the likelihood of an R0 resection is another subject of interest which can be examined in future studies. Patients with Stage IV disease and patients with positive margins after surgical resection should be highly considered for multimodal therapy to attempt to treat residual disease as best possible. Although this tumor is rare and the increased incidence may not be immediately clinically significant, this could change over time and this rare cancer may be seen more often in coming decades.
Limitations
This study is limited by its retrospective nature. Furthermore, the use of large databases creates additional limitations. First, re-review of pathologic specimens or cross-sectional imaging is not possible with NCDB data. Additionally, while we examined the chemotherapy pattern between PACC and PDAC using NCBD data, we could not provide comparable results using the SEER database as SEER does not have chemotherapy sequencing data (with respect to surgery) before 2019. Furthermore, the NCDB and SEER databases do not provide granular details on the chemotherapy (i.e., chemotherapy types, dose, cycles, and duration). The NCDB database is also limited to CoC approved facilities, leaving approximately 30% of cancer diagnosis uncaptured in our analysis. Another limitation of this study is determining the clinical significance of the increasing incidence. PACC is a very rare malignancy; therefore, the increase in incidence may be statistically significant but not clinically relevant for a period of time.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used are derived from a de-identified NCDB participant user file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methods or the conclusions drawn from these data by the investigators. The authors gratefully acknowledge the support of the Mayo Clinic Department of Surgery and the Kern Center for the Science of Health Care Delivery as substantial contributors of in-kind resources to the project.
Funding: The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery provides salary support for Dr. Habermann and in kind material support for Dr. Bergquist. No specific grants are associated with this work.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-21-1). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jain D. Acinar cell carcinoma [Internet]. Pathology Outlines - PathologyOutlines.com. [cited 2019 Mar 23]. Available online: https://www.pathologyoutlines.com/topic/pancreasacinar.html
- La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne) 2015;2:41. [Crossref] [PubMed]
- Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673-8. [Crossref] [PubMed]
- Al-Hader A, Al-Rohil RN, Han H, et al. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World Journal of Gastroenterology 2017;23:7945-51. [Crossref] [PubMed]
- Luo G, Fan Z, Gong Y, et al. Characteristics and Outcomes of Pancreatic Cancer by Histological Subtypes. Pancreas 2019;48:817-822. [Crossref] [PubMed]
- Wisnoski NC, Townsend CM Jr, Nealon WH, et al. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery 2008;144:141-8. [Crossref] [PubMed]
- Wang Y, Wang S, Zhou X, et al. Acinar cell carcinoma: a report of 19 cases with a brief review of the literature. World J Surg Oncol 2016;14:172. [Crossref] [PubMed]
- Matos JM, Schmidt CM, Turrini O, et al. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg 2009;13:1495-502. [Crossref] [PubMed]
- Seth AK, Argani P, Campbell KA, et al. Acinar cell carcinoma of the pancreas: An institutional series of resected patients and review of the current literature. J Gastrointest Surg 2008;12:1061-7. [Crossref] [PubMed]
- Chaudhary P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J Surg 2015;77:226-231. [Crossref] [PubMed]
- National Cancer Institute. Overview of the SEER Program. Available online: http://seer.cancer.gov/about/. 2010.
- Raval M V, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol 2009;99:488-90. [Crossref] [PubMed]
- Jiao Y, Yonescu R, Offerhaus GJ, et al. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol 2014;232:428-35. [Crossref] [PubMed]
- Thompson ED, Wood LD. Pancreatic Neoplasms With Acinar Differentiation: A Review of Pathologic and Molecular Features. Arch Pathol Lab Med 2020;144:808-15. [Crossref] [PubMed]
- FDA. Highlights of Prescribing Information [Internet]. [cited 2021 Mar 16]. Available online: www.fda.gov/medwatch.
- Luchini C, Paolino G, Mattiolo P, et al. KRAS wild-type pancreatic ductal adenocarcinoma: molecular pathology and therapeutic opportunities. J Exp Clin Cancer Res 2020;39:227. [Crossref] [PubMed]
- Toll AD, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: clinical and cytomorphologic characteristics. Korean J Pathol 2013;47:93-9. [Crossref] [PubMed]
- Frampas E, David A, Regenet N, et al. Pancreatic carcinoma: Key-points from diagnosis to treatment. Diagnostic and Interventional Imaging 2016;97:1207-23. [Crossref] [PubMed]
- Chiou YY, Chiang JH, Hwang JI, et al. Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr 2004;28:180-6. [Crossref] [PubMed]
- Lim JH, Chung KB, Cho OK, et al. Acinar cell carcinoma of the pancreas. Ultrasonography and computed tomography findings. Clin Imaging 1990;14:301-4. [Crossref] [PubMed]
Cite this article as: Yonkus JA, Bergquist JR, Alva-Ruiz R, Ivanics T, Habermann EB, Abdelrahman AM, Grotz TE, Cleary SP, Smoot RL, Nagorney DM, Kendrick ML, Halfdanarson TR, Truty MJ. A national database analysis of acinar cell carcinoma of the pancreas, a histologically, epidemiologically, and biologically distinct entity increasing in incidence. Ann Pancreat Cancer 2021;4:3.


