
Case series: FDG-avid lymphadenopathy during oncologic staging of pancreatic adenocarcinoma after COVID-19 vaccination
Introduction
Despite declining cases of COVID-19 in the United States and the ramping up of vaccine administration, clinicians continue to adapt their logistical and clinical practices. Of particular interest to clinicians taking care of oncology patients, the reported sequela of lymphadenopathy on imaging and clinical examination after recent COVID-19 vaccination creates a unique challenge when staging and surveilling cancer patients. Per the Centers for Disease Control and Prevention, over 140 million doses of COVID-19 vaccine have been administered with millions more anticipated. Unsolicited self-reporting of lymphadenopathy after the 2nd Moderna vaccination was seen in approximately 15% of people who received the vaccine (1). Lymphadenopathy has been reported on CT scans, MRI, 18FDG PET/CT scans, mammography, and ultrasonography in the neck, axilla, mediastinum, and supraclavicular fossa (2-4). This has already started confounding screening, treatment, and surveillance for patients with breast cancer, lung cancer, lymphoma, and head and neck cancers (5,6). Vaccine associated FDG-avid lymphadenopathy has been reported in 45.8% of patients who received the 2nd dose of the Pfizer vaccine (7). Staging and treatment of pancreatic adenocarcinoma also has the potential to be complicated by post-COVID-19 vaccination lymphadenopathy.
Adenocarcinoma has characteristic patterns of metastasis depending on the location of the primary tumor (8,9). The most common sites of metastasis for pancreatic adenocarcinoma are the liver and peritoneal cavity while the lungs, bones, and brain are less common. Spread to supraclavicular lymph nodes is rare; however, it has been reported on multiple occasions and cannot be overlooked if lymphadenopathy is present on physical exam or imaging (10,11). Since both metastatic disease and post-vaccine lymphadenopathy can present in the supraclavicular fossa, patients can be mischaracterized as having metastatic disease or indeterminant nodes. This can lead to oncologic upstaging, unnecessary changes of treatment, or delay of appropriate treatment.
Treating pancreatic adenocarcinoma with neoadjuvant chemoradiation for advanced disease prior to surgery has shown to improve survival (12,13). Patients undergoing staging, monitoring of treatment response, and surveillance require routine imaging. At any point during this process the finding of lymphadenopathy can change the oncologic stage and affect the treatment plan. We present three cases of FDG-avid lymphadenopathy after COVID-19 vaccine administration in patients at various stages of treatment for pancreatic adenocarcinoma. Management of lymphadenopathy varied depending on the patients’ treatment. Different management strategies included repeat imaging at follow-up, ultrasound guided fine needle aspiration (FNA), and excisional lymph node biopsy for histopathology.
We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/apc-21-8).
Case presentation
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Mayo Clinic (21-003481). Written informed consent for publication was obtained from the patients.
Case 1
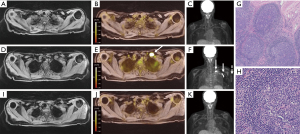
Patient 1 is a 57-year-old female diagnosed with locally advanced pancreatic adenocarcinoma undergoing treatment with FOLFIRINOX. She subsequently presented to our institution for reevaluation. Abdominal CT showed a 2.9 cm × 2.7 cm × 2.6 cm infiltrative mass in the pancreatic head with significant vascular involvement. 18FDG PET/CT did not show signs of distant metastatic disease. Neoadjuvant chemotherapy agents were switched, and restaging imaging was performed 6 months later. Chest CT with IV contrast and 18FDG PET/MRI 6 months later showed significant tumor regression, however, new FDG-avid left supraclavicular and axillary lymphadenopathy was present (Figure 1A-1F). She received the 2nd dose of the Pfizer COVID-19 vaccine in the left deltoid 12 days prior to chest CT and 13 days prior to 18FDG PET/MRI. The left supraclavicular node was the largest measuring 2.0 cm with an SUVmax of 20.9. Decision was made to proceed with radiation and surgery if the new FDG-avid nodes were determined to be non-malignant. The patient subsequently underwent ultrasound guided FNA of the left supraclavicular lymph node which was negative for malignancy and demonstrated appropriate admixture of lymphocytes. Excisional biopsy of the left supraclavicular lymph node was then performed in order to rule out metastatic disease. Histopathology showed reactive follicular hyperplasia without features of metastatic carcinoma (Figure 1G,1H). This was consistent with histological findings reported by Özütemiz et al. The patient proceeded with pre-surgical radiotherapy. Lymphadenopathy in the axilla and subpectoral region resolved on repeat 18FDG PET/MRI three months later (Figure 1I-1K).
Case 2
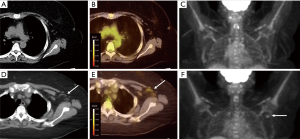
Patient 2 is a 49-year-old female with locally advanced pancreatic adenocarcinoma undergoing treatment with gemcitabine and paclitaxel. During initial staging, there were no signs of metastatic disease on imaging. She subsequently presented to our institution for reevaluation. New imaging was obtained that showed partial response of the pancreatic mass without metastatic disease, however, 18FDG PET/CT showed new nodal enlargement with SUVmax of 2.21 in the left axilla when compared to baseline imaging (Figure 2). The patient received their 2nd Moderna vaccination in the left deltoid muscle 33 days prior to 18FDG PET/CT. From a surgical standpoint, there was not adequate tumor regression so continued neoadjuvant chemotherapy was required before proceeding to radiation and surgery. With a high probability of the axillary lymphadenopathy being due to recent COVID-19 vaccination; the decision was made to continue with planned imaging in 3 months for cancer restaging without further interrogation of the lymph nodes.
Case 3
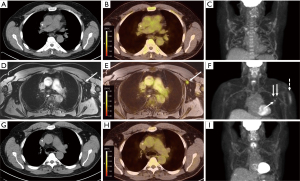
Patient 3 is a 62-year-old male with a diagnosis of locally advanced pancreatic adenocarcinoma undergoing neoadjuvant chemotherapy with gemcitabine and paclitaxel. Initial 18FDG PET/CT showed a 6.2 cm hypermetabolic mass in the body of the pancreas without signs of metastatic disease. The patient subsequently presented to our institution for reevaluation. The patient had repeat imaging for restaging that showed mild decrease in pancreatic tumor size on abdominal CT. 18FDG PET/MRI did not show any peritoneal disease; however, there was new FDG-avid lymph nodes in the left axilla and neck along with left deltoid muscle uptake with highest SUVmax of 3.45 (Figure 3A-3F). The patient received the Johnson and Johnson vaccine in the left deltoid 11 days prior to 18FDG PET/MRI. The decision was made to observe the post-vaccine lymphadenopathy and continue neoadjuvant chemotherapy and planned restaging in order to maximize radiologic and metabolic response. Repeat imaging two months later showed anatomic and metabolic resolution of the lymphadenopathy (Figure 3G-3I).
Discussion
Post-COVID-19 vaccine associated lymphadenopathy has created a new challenge for management of oncology patients. While post-vaccination lymphadenopathy is not a new concept, widely distributed vaccine implementation at this scale has not occurred in the U.S. in the last several decades (14,15). Clinicians will inevitably see an increase of patients with post-vaccination lymphadenopathy as COVID-19 vaccine distribution continues. We provide three examples of management of this side effect in patients diagnosed with pancreatic adenocarcinoma at different time points of treatment that will hopefully help to guide future management of patients in these scenarios.
Case 1 demonstrated management of the side effect at a critical juncture of the patient’s treatment. In this case, the decision was made to definitively rule out metastatic disease before proceeding with surgery thus FNA and subsequently excisional biopsy were performed in order to obtain a histopathologic diagnosis. While it did add two additional procedures that have their own associated risks and costs, performing a pancreaticoduodenectomy on the patient if the lymphadenopathy was actually metastatic disease would have been inappropriate treatment for this patient (16). An alternative approach could have been to reimage later to see if the lymphadenopathy resolves to a level that there is no concern for malignancy. However, the risk to this approach is further delaying treatment of the tumor. Proceeding to surgery without additional imaging or histopathological diagnosis may also be an option in patients with a high probability of benign reactive lymphadenopathy. The type and location of the primary tumor are also key factors to take into consideration.
Clinical observation and repeat imaging appear to be a reasonable approach for patients that are not imminent surgical candidates, however, timing of this approach is evolving. McIntosh et al. recommended waiting at least 2 weeks after vaccination for 18PET FDG imaging, however, more recent studies have shown PET-avidity to persist in some patients up to 10 weeks after vaccination (17,18). In Case 2 and 3, the lymphadenopathy was observed and reimaged with planned tumor restaging since they were continuing neoadjuvant chemotherapy and were not candidates for imminent surgery. In cases where a definitive histopathologic diagnosis is not needed immediately and there is a low probability of malignant metastasis, a reasonable option is observation and reevaluation on future restaging imaging. Another strategy to take into consideration is timing of the COVID-19 vaccination in patients that have not received their vaccination yet. The risks and benefits should be discussed with a team approach involving the patient as the risk of COVID infection is higher in immunosuppressed patients and can have increased morbidity and mortality in this population (19-21). Because of this, most cancer centers do not recommend delaying vaccination (22). However, proactively obtaining initial imaging prior to vaccination may help to prevent upstaging in the initial evaluation.
Lymphadenopathy in oncology patients after recent COVID-19 vaccination should be managed with a multi-disciplinary team that includes the surgeon, oncologist, primary care provider, and radiologist. Factors such as surgical candidacy, likelihood of reactive lymphadenopathy, probability of metastatic disease, and risk of delaying surgery or treatment should be taken into consideration. Unnecessary imaging and procedures should be avoided in cases where treatment would not be changed based on the histopathologic diagnosis of the lymphadenopathy. The treatment team should have accurate documentation of vaccine administration so that clinical findings can be put into proper context. Additional studies on the duration of post-vaccination lymphadenopathy in oncology patients need to be done in order to help guide management in this patient population. We recognize that this report only demonstrates the management of three patients in this situation. With limited reporting, lack of randomized controlled trials, and lack of more powerful studies, clinicians must make decisions based on their knowledge and experience with the patient’s health at the forefront.
Acknowledgments
The authors would like to thank all the providers that helped to gather information and provided care for the patients in the study. We would also like to thank all the patients for their consent for publication.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/apc-21-8
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apc-21-8). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of the Mayo Clinic (21-003481). Written informed consent for publication was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403-16. [Crossref] [PubMed]
- Eifer M, Eshet Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology 2021;299:E248 [Crossref] [PubMed]
- Hanneman K, Iwanochko RM, Thavendiranathan P. Evolution of Lymphadenopathy at PET/MRI after COVID-19 Vaccination. Radiology 2021;299:E282 [Crossref] [PubMed]
- Mehta N, Sales RM, Babagbemi K, et al. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging 2021;75:12-5. [Crossref] [PubMed]
- Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 2021;300:E296-300. [Crossref] [PubMed]
- McIntosh LJ, Bankier AA, Vijayaraghavan GR, et al. COVID-19 Vaccination-Related Uptake on FDG PET/CT: An Emerging Dilemma and Suggestions for Management. AJR Am J Roentgenol 2021;217:975-83. [Crossref] [PubMed]
- Cohen D, Krauthammer SH, Wolf I, et al. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging 2021;48:1854-63. [Crossref] [PubMed]
- Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer 2006;106:1624-33. [Crossref] [PubMed]
- van Overhagen H, Brakel K, Heijenbrok MW, et al. Metastases in supraclavicular lymph nodes in lung cancer: assessment with palpation, US, and CT. Radiology 2004;232:75-80. [Crossref] [PubMed]
- Soman AD, Collins JM, DePetris G, et al. Isolated supraclavicular lymph node metastasis in pancreatic adenocarcinoma: a report of three cases and review of the literature. JOP 2010;11:604-9. [PubMed]
- Saif MW, Hotchkiss S, Brennan M, et al. Pancreatic adenocarcinoma with supraclavicular lymph node metastasis: is this the Virchow's node? JOP 2011;12:66-7; author reply 70. [PubMed]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123-32. [Crossref] [PubMed]
- Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol 2010;5:510-6. [Crossref] [PubMed]
- Panagiotidis E, Exarhos D, Housianakou I, et al. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol 2010;20:1251-3. [Crossref] [PubMed]
- Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science 2009;325:1705-8. [Crossref] [PubMed]
- Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg 2008;247:456-62. [Crossref] [PubMed]
- Eshet Y, Tau N, Alhoubani Y, et al. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology 2021;300:E345-7. [Crossref] [PubMed]
- Shirone N, Shinkai T, Yamane T, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med 2012;26:248-52. [Crossref] [PubMed]
- Al-Quteimat OM, Amer AM. The Impact of the COVID-19 Pandemic on Cancer Patients. Am J Clin Oncol 2020;43:452-5. [Crossref] [PubMed]
- Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218-23. [Crossref] [PubMed]
- Mehta V, Goel S, Kabarriti R, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov 2020;10:935-41. [Crossref] [PubMed]
- Lehman CD, D'Alessandro HA, Mendoza DP, et al. Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists Across Specialties. J Am Coll Radiol 2021;18:843-52. [Crossref] [PubMed]
Cite this article as: Nathan JM, Navin PJ, Truty MJ, Dy BM. Case series: FDG-avid lymphadenopathy during oncologic staging of pancreatic adenocarcinoma after COVID-19 vaccination. Ann Pancreat Cancer 2021;4:9.


