
Blood transfusion is associated with worse outcomes following pancreatic resection for pancreatic adenocarcinoma
Introduction
Pancreatectomy remains the only potentially curative therapy for patients with pancreatic ductal adenocarcinoma (PDAC) (1). Pancreatic resection can be performed with low mortality, but remains complicated by significant morbidity, which occurs in 40–60% of patients. Following pancreatic resection, pancreatic fistula and delayed gastric emptying are most frequent complications that arise and have been the focus of numerous studies (2). Also common, perioperative allogeneic blood transfusion (PBT) in the setting of pancreatectomy has gained interest due to the association with perioperative morbidity and increased propensity for recurrence and death (3-8). Furthermore, PBT has been identified as a clinicopathological factor associated with failure to complete adjuvant therapy following resection for PDAC (7). The exact mechanism underlying these findings is unclear; however, the adverse effect on oncologic outcome was initially proposed in 1981 and may be consequent to the immunomodulatory effect from transfusions (9,10). Subsequent investigations suggest clonal deletion of specific immune cells leading to depressed cell-mediated immunity and immunosuppression as putative mechanisms; however, the pathophysiology is poorly understood (10).
Existing literature indicates that 27–68% of patients require PBT following pancreatectomy, with a recent meta-analysis identifying 46.5% of patients receiving at least one unit of blood in the perioperative period (1,11,12). The complex nature of these operations and operative and/or postoperative blood loss oftentimes requires transfusion of blood products either intraoperatively or postoperatively; but the precise threshold should be appropriately defined to avoid indiscriminate transfusion practices (13). This is especially more pertinent as surgeons employ more aggressive surgical techniques (14-17). In many studies, blood is provided in circumstances of intraoperative blood loss concomitant with hemodynamic changes or postoperative anemia (hemoglobin <7–8 mg/dL) (13,18). While there are different transfusion triggers reported, a lower hemoglobin threshold (7–8 mg/dL) results in reduced red blood cell transfusion without increased morbidity or mortality compared to transfusion at higher hemoglobin levels (19). Limiting transfusions may be beneficial as an association between PBT and cancer recurrence or decreased survival has been reported (4-6). However, there exists controversy regarding the reported negative impact of PBT on overall survival (OS), with some authors suggesting worse outcome is confounded by the clinical circumstances requiring the transfusions (11,20).
Therefore, we aimed to study impact on survival of PBT in patients undergoing pancreatic surgery at a high-volume single institution. This is the largest study to date that examines the effect of PBT on oncologic outcome for PDAC. We present the following article in accordance with the STROBE reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-21-11/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review boards of Johns Hopkins University (No. 00092443) and individual consent for this retrospective analysis was waived.
Two prospectively maintained institutional databases managed by the Department of Surgery and Department of Anesthesiology were used to identify patients with PDAC undergoing curative-intent pancreatectomy at Johns Hopkins Hospital from 2009 to 2015. Patients were excluded if they underwent a macroscopically positive resection (R2) or had metastatic disease. In addition, patients who experienced in-hospital mortality were also excluded from the analysis.
Variables and definitions
Data on clinicopathological variables were extracted from the databases. Patients’ electronic medical records were reviewed to collect missing data. Demographic variables included age, gender, and race. Preoperative laboratory values included serum albumin, total bilirubin, hemoglobin, and chemistry panel. Medical comorbidities included obesity, diabetes, cardiac disease, pulmonary disease, chronic kidney disease, and American Society of Anesthesiologist (ASA) status.
PBT was defined as transfusion of any amount or type of blood product that were transfused during the operation or the 30 days postoperative period. Further categorization included intraoperative versus postoperative blood transfusion and number of units transfused. Restrictive use of PBT was defined as any transfusion at a hemoglobin of less than 8 g/dL, whereas liberal use was defined as transfusion performed at a hemoglobin of greater than 8d/dL. Patients were stratified by PBT and clinicopathological variables were analyzed. The primary outcome measure was median OS, defined as the duration from date of surgery until death. The 8th edition of the American Joint Committee on Cancer (AJCC) for pancreatic cancer staging was used. This study was approved by the institutional review boards at Johns Hopkins Hospital.
Statistical analysis
Categorical variables were reported as frequencies and percentages while continuous variables were reported as means and standard deviations or median and interquartile ranges. Statistical significance was determined by Chi2 or Fisher’s exact testing (categorical variables) or Wilcoxon rank-sum testing (continuous variables). OS was calculated by Kaplan-Meier method and differences between curves were investigated with the log-rank test. Univariable and multivariable logistical regression models were used to determine association between clinicopathological factors and OS. Factors demonstrating a P value of <0.2 on univariate model were included in the multivariable Cox proportional hazard regression model. Hazard ratio (HR) and 95% confidence interval (CI) for variables included in the multivariable model were reported. A P value <0.05 was considered statistically significant, and all analyses were performed in STATA version 14.1 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
A total of 546 patients were identified of which 238 patients (43%) received PBT. The mean age was 64.5±10.5 years and a 52.9% (N=289) were male. Details on the clinicopathological characteristics are summarized in Table 1. The median number of units transfused in patients requiring PBT was 2 units (IQR: 2–5). Patients undergoing PBT were more likely to be greater than 65 years of age, non-white race, ASA class III/IV, and were more likely to have coagulopathy, or anemia (all P<0.05, Table 2). Examining operative variables demonstrated higher likelihood of greater operative blood loss (P<0.001) in the PBT group (Table 2). All remaining patient, operative and pathologic characteristics were not significantly different.
Table 1
| Variables | Number (%) |
|---|---|
| Age, ≥65 years | 262 (47.9) |
| Gender, female | 257 (47.1) |
| Race | |
| White | 497 (91.0) |
| African American | 32 (5.9) |
| Other | 17 (3.1) |
| ASA, III/IV | 394 (72.2) |
| Diabetes mellitus, present | 108 (19.8) |
| Hypertension, present | 292 (53.5) |
| Coagulopathy*, present | 12 (2.2) |
| Anemia, present | 212 (38.8) |
| Past abdominal surgery, performed | 128 (23.4) |
| Abdominal pain, present | 200 (36.6) |
| Jaundice, present | 234 (42.9) |
| Weight loss, present | 161 (29.5) |
| Neoadjuvant chemotherapy, performed | 171 (31.3) |
| Type of surgery | |
| Pancreaticoduodenectomy | 439 (80.4) |
| Distal pancreatectomy | 91 (16.7) |
| Total pancreatectomy | 16 (2.9) |
| Vascular resection, performed | 87 (15.9) |
| Estimate blood loss, >500 mL | 319 (58.4) |
| AJCC T-stage, III/IV | 271 (49.6) |
| Nodal disease, present | 352 (64.5) |
| Number of harvested nodes, ≥20 | 280 (51.3) |
| Margin status, positive | 133 (24.4) |
| Grade of tumor differentiation | |
| Well/moderate | 298 (54.6) |
| Poor/undifferentiated | 221 (40.5) |
| Lymphovascular invasion, present | 286 (52.4) |
| Perineural invasion, present | 427 (78.2) |
| Adjuvant chemotherapy, performed | 239 (43.8) |
*, on multivariate analysis there was a direct association with coagulopathy and need for transfusion. PDAC, pancreatic ductal adenocarcinoma; ASA, American Society of Anesthesiologists; AJCC, American Joint Committee on Cancer.
Table 2
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| No transfusion (N=308) | Receipt of transfusion (N=238) | P value | Odds ratio (95% CI) | P value | ||
| Age, ≥65 years | 129 (41.9) | 133 (43.2) | 0.001 | 1.01 (1.00–1.02) | <0.001 | |
| Gender, female | 153 (49.7) | 104 (33.8) | 0.165 | |||
| Race | 0.006 | |||||
| White | 290 (94.2) | 207 (67.2) | Ref. | – | ||
| African American | 14 (4.5) | 18 (5.8) | 2.39 (0.90–6.33) | 0.078 | ||
| Other | 4 (1.3) | 13 (4.2) | 9.37 (2.31–38.02) | 0.002 | ||
| ASA, III/IV | 196 (63.6) | 198 (64.3) | <0.001 | 1.82 (1.08–3.08) | 0.024 | |
| Diabetes mellitus, present | 58 (18.8) | 50 (16.2) | 0.527 | |||
| Hypertension, present | 157 (51.0) | 135 (43.8) | 0.182 | |||
| Coagulopathy*, present | 0 (0.0) | 12 (3.9) | <0.001 | – | <0.001 | |
| Anemia, present | 57 (18.5) | 155 (50.3) | <0.001 | 8.86 (5.47–14.34) | <0.001 | |
| Past abdominal surgery, performed | 64 (20.8) | 64 (20.8) | 0.095 | |||
| Abdominal pain, present | 106 (34.4) | 94 (30.5) | 0.222 | |||
| Jaundice, present | 123 (39.9) | 111 (36.0) | 0.117 | |||
| Weight loss, present | 86 (27.9) | 75 (24.4) | 0.362 | |||
| Neoadjuvant chemotherapy, performed | 79 (25.6) | 92 (29.9) | 0.001 | 1.38 (0.85–2.25) | 0.186 | |
| Type of surgery | 0.434 | |||||
| Pancreaticoduodenectomy | 246 (79.9) | 193 (62.7) | ||||
| Distal pancreatectomy | 55 (17.9) | 36 (11.7) | ||||
| Total pancreatectomy | 7 (2.3) | 9 (2.9) | ||||
| Vascular resection, performed | 33 (10.7) | 54 (17.5) | <0.001 | 1.46 (0.78–2.73) | 0.235 | |
| Estimate blood loss, >500 mL | 133 (43.2) | 186 (60.4) | <0.001 | 1.00 (1.00–1.00) | <0.001 | |
| AJCC T-stage, III/IV | 145 (47.1) | 126 (40.9) | 0.174 | |||
| Nodal disease, present | 205 (66.6) | 147 (47.7) | 0.246 | |||
| Number of harvested nodes, ≥20 | 166 (53.9) | 114 (37.0) | 0.164 | |||
| Margin status, positive | 64 (20.8) | 69 (22.4) | 0.027 | 1.333 (0.78–2.26) | 0.289 | |
| Grade of tumor differentiation | 0.198 | |||||
| Well/moderate | 176 (57.1) | 122 (39.6) | ||||
| Poor/undifferentiated | 118 (38.3) | 103 (33.4) | ||||
| Lymphovascular invasion, present | 156 (50.6) | 130 (42.2) | 0.357 | |||
| Perineural invasion, present | 242 (78.6) | 185 (60.1) | 0.814 | |||
| Adjuvant chemotherapy, performed | 135 (43.8) | 104 (33.8) | 0.975 | |||
*, on multivariate analysis there was a direct association with coagulopathy and need for transfusion. PDAC, pancreatic ductal adenocarcinoma; Ref, Reference; ASA, American Society of Anesthesiologists; AJCC, American Joint Committee on Cancer.
Hemoglobin nadir and PBT
A total of 157 patients (28.8%) required transfusions intraoperatively of whom 94 (17.2%) required both intraoperative and postoperative transfusions. An additional, 81 patients (14.8%) required postoperative transfusions but no intraoperative transfusions. Of the patients who received intraoperative transfusions, 63.5% (N=40) received transfusions without evidence of hemoglobin nadir <8 g/dL. This is likely representative of intraoperative loss with transfusion used as a strategy to provide colloid resuscitation in the setting of relative hemodynamic instability. A restrictive approach was more commonly used in the postoperative setting where a majority of patients (N=69, 85.2%) requiring postoperative transfusions were transfused at a hemoglobin nadir of <8 g/dL. While a liberal approach was more common in the intraoperative setting, patients who did receive intraoperative transfusions were less likely to receive postoperative transfusion (40.3% vs. 74.4%, P<0.001).
OS
The median OS for the entire cohort was 24.8 months. PBT was associated with a poorer median OS in comparison to patients who did not receive PBT (17.1 vs. 27.4 months, P<0.001; Figure 1). Of the patients that received PBT, a majority (N=179, 75.2%) received two or more units of blood, while fewer patients (N=59, 24.8%) were transfused only one unit in the perioperative period. When stratifying based on number of units transfused, those transfused two or more units of blood experienced shorter median survival compared to no transfusion (15.9 vs. 27.4 months, P<0.001); there was a trend towards worse survival in comparison to one unit (Figure 2).
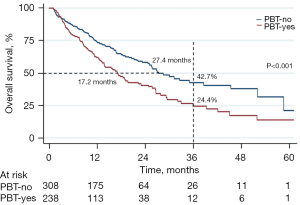
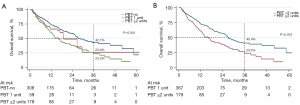
Factors associated with OS
In the univariable analysis, several factors were associated with worse OS including older age, male gender, weight loss, estimated blood loss, AJCC T-stage and N-stage, tumor size greater than 3 cm, number of harvested nodes, number of positive nodes, margin-positive resection, lymphovascular invasion, poor tumor differentiation, intra-operative transfusion and transfusion during hospitalization. On multivariable analysis, PBT was independently associated with poorer OS (HR =1.55, 95% CI: 1.07–2.25, P=0.020). Additional factors negatively impacting OS were age greater than 65, male gender, presence of weight loss, less than 20 harvested nodes, lymphovascular invasion, positive margin status, AJCC T-stage III or IV and tumor size greater than 3 cm (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| N (%) | OS (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 0.017 | 0.045 | ||||
| <65 years | 284 (52.0) | 38.2 (29.0–47.2) | Ref. | |||
| ≥65 years | 262 (48.0) | 31.2 (22.5–40.3) | 1.01 (1.00–1.01) | |||
| Gender | 0.001 | 0.010 | ||||
| Male | 289 (52.9) | 21.9 (13.9–31.1) | Ref. | |||
| Female | 257 (47.1) | 47.7 (38.6–56.4) | 0.69 (0.52–0.92) | |||
| Race | 0.800 | |||||
| White | 497 (91.0) | 34.7 (28.1–41.3) | ||||
| African American | 14 (2.6) | 30.4 (5.9–60.7) | ||||
| Other | 35 (6.4) | – | ||||
| ASA | 0.125 | |||||
| I/II | 152 (52.6) | 37.0 (25.2–48.8) | ||||
| III/IV | 394 (47.4) | 33.9 (26.3–41.6) | ||||
| Diabetes mellitus | 0.694 | |||||
| Absent | 438 (80.2) | 37.3 (30.1–44.6) | ||||
| Present | 108 (19.8) | 26.0 (14.2–39.5) | ||||
| Hypertension | 0.539 | |||||
| Absent | 254 (46.5) | 36.7 (27.8–45.7) | ||||
| Present | 292 (53.5) | 32.6 (23.6–41.9) | ||||
| Coagulopathy | 0.837 | |||||
| Absent | 534 (97.8) | 35.1 (28.7–41.6) | ||||
| Present | 12 (2.2) | – | ||||
| Anemia | 0.060 | |||||
| Absent | 327 (60.7) | 28.1 (18.5–38.6) | ||||
| Present | 212 (39.3) | 38.8 (30.4–46.9) | ||||
| Past abdominal surgery | 0.392 | |||||
| Not performed | 418 (76.6) | 35.0 (27.7–42.4) | ||||
| Performed | 128 (23.4) | 33.9 (20.9–47.4) | ||||
| Abdominal pain | 0.741 | |||||
| Absent | 346 (63.4) | 36.7 (28.6 –44.9) | ||||
| Present | 200 (36.6) | 33.9 (20.9 –47.4) | ||||
| Jaundice | 0.450 | |||||
| Absent | 312 (57.1) | 35.6 (26.9–44.4) | ||||
| Present | 234 (42.9) | 33.6 (24.3–43.1) | ||||
| Weight loss | 0.008 | 0.009 | ||||
| Absent | 385 (70.5) | 37.2 (29.3–45.1) | Ref. | |||
| Present | 161 (29.5) | 29.0 (18.7–40.2) | 1.45 (1.09–1.92) | |||
| Neoadjuvant chemotherapy | 0.088 | |||||
| Not performed | 375 (68.7) | 37.8 (30.4–45.2) | ||||
| Performed | 171 (31.3) | 24.9 (13.2–38.5) | ||||
| Type of surgery | 0.428 | |||||
| Pancreaticoduodenectomy | 439 (80.4) | 33.1 (26.1–40.3) | ||||
| Distal pancreatectomy | 91 (16.7) | 41.8 (26.4–56.4) | ||||
| Total pancreatectomy | 16 (2.9) | 57.2 (27.1–78.7) | ||||
| Vascular resection | 0.324 | |||||
| Not performed | 459 (84.1) | 36.5 (29.6–43.4) | ||||
| Performed | 87 (15.9) | 22.7 (7.9–41.9) | ||||
| Estimate blood loss | 0.002 | 0.409 | ||||
| ≤500 mL | 207 (39.3) | 49.0 (38.6–58.6) | Ref. | |||
| >500 mL | 319 (60.7) | 27.1 (19.5 –35.3) | 1.00 (0.99–1.01) | |||
| Intraoperative transfusion | 0.005 | 0.488 | ||||
| Not performed | 389 (71.3) | 40.3 (32.6 – 47.8) | Ref. | |||
| Performed | 157 (28.7) | 20.9 (11.3 –32.6) | 0.87 (0.58–1.29) | |||
| AJCC T-stage | <0.001 | 0.003 | ||||
| I/II | 275 (50.3) | 47.3 (37.9–55.9) | Ref. | |||
| III/IV | 271 (49.6) | 20.8 (13.2–29.6) | 1.54 (1.15–2.06) | |||
| Nodal disease | 0.001 | 0.509 | ||||
| Absent | 194 (35.5) | 47.7 (36.9–57.7) | Ref. | |||
| Present | 352 (64.5) | 27.1 (19.5–35.2) | 1.13 (0.79–1.63) | |||
| Number of harvested nodes | 0.022 | 0.003 | ||||
| <20 | 266 (48.7) | 30.5 (21.8–39.6) | Ref. | |||
| ≥20 | 280 (51.3) | 38.8 (29.7–47.8) | 0.98 (0.97–0.99) | |||
| Margin status | <0.001 | 0.014 | ||||
| Negative | 413 (75.6) | 39.8 (32.2–47.3) | Ref. | |||
| Positive | 133 (24.4) | 19.3 (9.3–32.0) | 1.45 (1.08–1.94) | |||
| Grade of tumor differentiation | 0.019 | 0.093 | ||||
| Well/moderate | 298 (57.5) | 38.7 (29.7–47.7) | Ref. | |||
| Poor/undifferentiated | 220 (42.5) | 29.4 (20.5 –38.8) | 1.23 (0.96–1.64) | |||
| Lymphovascular invasion | <0.001 | 0.011 | ||||
| Absent | 260 (47.6) | 45.3 (35.3 –54.8) | Ref. | |||
| Present | 286 (52.4) | 25.5 (17.7 –34.1) | 1.46 (1.09–1.96) | |||
| Perineural invasion | 0.286 | |||||
| Absent | 119 (21.8) | 37.4 (22.9–51.9) | ||||
| Present | 427 (78.2) | 34.1 (27.0–41.2) | ||||
| Transfusion during hospitalization | <0.001 | 0.020 | ||||
| Not performed | 308 (56.4) | 42.7 (33.7–51.3) | Ref. | |||
| Performed | 238 (43.6) | 24.6 (16.0–33.8) | 1.55 (1.07–2.25) | |||
| Adjuvant chemotherapy | 0.223 | |||||
| Not performed | 307 (56.2) | 32.4 (23.3–41.8) | ||||
| Performed | 239 (43.8) | 36.9 (27.9–46.1) | ||||
P<0.05 is considered to be significant. PDAC, pancreatic ductal adenocarcinoma; Ref, reference; ASA, American Society of Anesthesiologists; AJCC, American Joint Committee on Cancer; OS, overall survival.
Discussion
This study evaluated the impact of PBT on survival of patients with resected PDAC. Receipt of PBT was independently associated with worse survival. In terms of number of units transfused, PBT of two units or more was significantly associated with worse survival. A majority of studies evaluating the impact of PBT on survival in patients with PDAC are multi-institutional studies. This study to date is the largest single-institution study demonstrating the deleterious effects of PBT on long-term outcomes in these patients, with an absolute difference of 10 months in median OS. Evaluation of a large group of patients undergoing resection at a single institution helped reduce the bias introduced by confounders, such as inter-institution variability in transfusion practices and bias introduced by degree of surgical aggression in advanced disease stage.
Studies on the impact of PBT on outcomes have been inconsistent in the past. Clark et al. focused on a relatively small sample size with limited OS survival (12 months) and reported no association between PBT and OS (11). In contrast, Kneuertz et al. explored a larger cohort in which the majority of patients received PBT and did find an association between PBT and survival (8). As in our study, their study was powered to a greater extent and permitted report of associated comorbid conditions with PBT requirement. Their report also investigated the impact of intraoperative transfusion, relative to postoperative transfusion, finding that intraoperative transfusion had no effect on RFS or OS, while postoperative transfusion resulted in earlier disease recurrence and reduced OS. This work was further carried forward with a meta-analysis by Mavros et al., concluding PBT was again associated with worse 5-year survival in aggregate (12). Finally, Sutton et al. explored the concept of a ‘dose effect’ for PBT whereby the intraoperative transfusion of more than 2 units was associated with poorer disease-free survival (DFS) (13). The primary driver of poorer survival in patients receiving PBT remains unclear. PBT has also been associated with failure to complete adjuvant chemotherapy (7). This is highly relevant to patients with PDAC, as adjuvant chemotherapy has a significant impact on survival (21-23). While Akahori et al. reported that patients receiving PBT were significantly less likely to complete adjuvant chemotherapy, our large cohort study fell short of confirming this association of a primary driver of outcome (7). Our results, alternatively, suggest that the impact of transfusions on survival are likely to be driven by considerations related to disease biology.
PBT is common after pancreatic resections. The rate of PBT at our institution between 2009 and 2015, 43%, is consistent with other contemporary reports (8,11,13,24,25). Given these rates of PBT and the documented adverse effect on outcome in our own patient cohort, we have initiated several implementation science studies to evaluate and adjust transfusion-related behaviors. This work resulted in a decrease in transfused units by over 50% (26). These data and our reported experience has added to the widespread push nationwide to implement evidence-based guidelines for appropriate transfusion triggers, particularly in patients undergoing hepatic and pancreatic resection (27). This need is highlighted by recent work by Ejaz et al. finding significant variability in the hemoglobin level prompting blood transfusion (27). Interestingly, in our study there was a trend towards older patients and those with more comorbidities being more likely to be transfused at a higher hemoglobin threshold. Our data here, and the recently reported work by Frank et al. from our institution, support a broader consensus that a restrictive transfusion practice is safe in complex hepatobiliary and pancreatic surgery (26).
Clinical decision making in the intraoperative period can be more nuanced—driven not only by objective hemoglobin assessment, but also by ongoing blood loss and assessment of hemodynamic stability or the need for alternative resuscitation strategies. The effect of transfusion at a variety of hemoglobin thresholds has been investigated in the setting of intraoperative transfusion (28,29). Again, older patients, those with more comorbidities, or those with starting hemoglobin less than 12 g/dL were more likely to receive an intraoperative blood transfusion. While this is not surprising, most patients who had a hemoglobin trigger of more than 10 g/dL and a target hemoglobin over 10 g/dL only received 1 or 2 units of blood during the operation and did not require additional transfusions. This indicates that some patients may not have required transfusions in the setting of a more restrictive transfusion practice (30-32). An additional strategy to minimizing the need for PBT across practice settings is a return to focusing on Halstedian principles of meticulous hemostasis during the intraoperative course (33). For example, Snyder and colleagues highlighted their recent experience in which the need for PBT during intraoperative vein resections during pancreatectomy was reduced to only 1/3rd of patients with excellent outcomes (34).
Though this study addresses many of the more recent limitations in practice variation posed by meta-analysis or large administrative datasets, the uniformity of practice is accompanied by several limitations. Our practice pattern is heavily biased towards the borderline resectable and locally advanced patient cohorts and, as such, selection bias with the accompanying increased proportion of patients requiring transfusion is a challenge to interpret in the context of wider datasets. The etiology that underlies the association between PBT and decreased survival remains obscured and is worthy of further study. We hypothesize from this work that disease biology, an immunomodulatory effect, and additional confounding factors may be the drivers of the phenomena described by these data. Though practice variation is minimized, the single-institution nature of this study is a relative limit on generalizability. While a multi-institutional study could overcome this limitation, multiple-institutional studies carry their own limitations, such as inter-institution variability in patient selection and patient management.
In conclusion, when evaluating our dataset for patients with mature oncologic follow-up, we find that nearly half of patients undergoing resection for PDAC required PBT. The receipt of PBT is associated with worse OS following resection. These data have served as the basis for ongoing work, both within our institution and more broadly, to adopt more restrictive practices for PBT. Potential benefits include improvements in perioperative safety, resource utilization and, as suggested by this work, superior cancer-specific long-term outcomes.
Acknowledgments
This work was presented at the Society of Surgical Oncology-70th Annual Cancer Symposium.
Funding: The study was supported by the Carolyn Pastorini Research Fund and the Drs. Keith and Valda Kaye Research Fund (both to Matthew J. Weiss).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-21-11/rc
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-21-11/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-21-11/coif). RAB serves as an unpaid Section Editor of Annals of Pancreatic Cancer from May 2021 to April 2023. MJW reports that the study was supported by the Carolyn Pastorini Research Fund and the Drs. Keith and Valda Kaye Research Fund. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review boards of Johns Hopkins University (No. 00092443) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Chalfin HJ, Liu JJ, Gandhi N, et al. Blood Transfusion is Associated with Increased Perioperative Morbidity and Adverse Oncologic Outcomes in Bladder Cancer Patients Receiving Neoadjuvant Chemotherapy and Radical Cystectomy. Ann Surg Oncol 2016;23:2715-22. [Crossref] [PubMed]
- Dresner SM, Lamb PJ, Shenfine J, et al. Prognostic significance of peri-operative blood transfusion following radical resection for oesophageal carcinoma. Eur J Surg Oncol 2000;26:492-7. [Crossref] [PubMed]
- Keller SM, Groshen S, Martini N, et al. Blood transfusion and lung cancer recurrence. Cancer 1988;62:606-10. [Crossref] [PubMed]
- Shiba H, Ishida Y, Wakiyama S, et al. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg 2009;13:1636-42. [Crossref] [PubMed]
- Akahori T, Sho M, Tanaka T, et al. Factors associated with failure to complete adjuvant chemotherapy in pancreatic cancer. Am J Surg 2016;211:787-92. [Crossref] [PubMed]
- Kneuertz PJ, Patel SH, Chu CK, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol 2011;18:1327-34. [Crossref] [PubMed]
- Gantt CL. Red blood cells for cancer patients. Lancet 1981;2:363. [Crossref] [PubMed]
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev 2007;21:327-48. [Crossref] [PubMed]
- Clark E, Connor S, Taylor MA, et al. Perioperative transfusion for pancreaticoduodenectomy and its impact on prognosis in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:472-7. [Crossref] [PubMed]
- Mavros MN, Xu L, Maqsood H, et al. Perioperative Blood Transfusion and the Prognosis of Pancreatic Cancer Surgery: Systematic Review and Meta-analysis. Ann Surg Oncol 2015;22:4382-91. [Crossref] [PubMed]
- Sutton JM, Kooby DA, Wilson GC, et al. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg 2014;18:1575-87. [Crossref] [PubMed]
- Habib JR, Kinny-Köster B, van Oosten F, et al. Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: Surgical planning with the "halo sign" and "string sign". Surgery 2021;169:1026-31. [Crossref] [PubMed]
- Kinny-Köster B, van Oosten F, Habib JR, et al. Mesoportal bypass, interposition graft, and mesocaval shunt: Surgical strategies to overcome superior mesenteric vein involvement in pancreatic cancer. Surgery 2020;168:1048-55. [Crossref] [PubMed]
- Diener MK, Mihaljevic AL, Strobel O, et al. Periarterial divestment in pancreatic cancer surgery. Surgery 2021;169:1019-25. [Crossref] [PubMed]
- Loos M, Kester T, Klaiber U, et al. Arterial Resection in Pancreatic Cancer Surgery: Effective After a Learning Curve. Ann Surg 2022;275:759-68. [Crossref] [PubMed]
- Bower MR, Ellis SF, Scoggins CR, et al. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol 2011;18:166-73. [Crossref] [PubMed]
- Carson JL, Carless PA, Hébert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA 2013;309:83-4. [Crossref] [PubMed]
- Müller SA, Mehrabi A, Rahbari NN, et al. Allogeneic blood transfusion does not affect outcome after curative resection for advanced cholangiocarcinoma. Ann Surg Oncol 2014;21:155-64. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Hallet J, Mahar AL, Tsang ME, et al. The impact of peri-operative blood transfusions on post-pancreatectomy short-term outcomes: an analysis from the American College of Surgeons National Surgical Quality Improvement Program. HPB (Oxford) 2015;17:975-82. [Crossref] [PubMed]
- Ejaz A, Spolverato G, Kim Y, et al. Impact of blood transfusions and transfusion practices on long-term outcome following hepatopancreaticobiliary surgery. J Gastrointest Surg 2015;19:887-96. [Crossref] [PubMed]
- Frank SM, Lo BD, Yesantharao LV, et al. Blood utilization and clinical outcomes in pancreatic surgery before and after implementation of patient blood management. Transfusion 2020;60:2581-90. [Crossref] [PubMed]
- Ejaz A, Spolverato G, Kim Y, et al. Identifying variations in blood use based on hemoglobin transfusion trigger and target among hepatopancreaticobiliary surgeons. J Am Coll Surg 2014;219:217-28. [Crossref] [PubMed]
- Spahn DR, Spahn GH, Stein P. Evidence base for restrictive transfusion triggers in high-risk patients. Transfus Med Hemother 2015;42:110-4. [Crossref] [PubMed]
- Ejaz A, Spolverato G, Kim Y, et al. Variation in triggers and use of perioperative blood transfusion in major gastrointestinal surgery. Br J Surg 2014;101:1424-33. [Crossref] [PubMed]
- Wehry J, Agle S, Philips P, et al. Restrictive blood transfusion protocol in malignant upper gastrointestinal and pancreatic resections patients reduces blood transfusions with no increase in patient morbidity. Am J Surg 2015;210:1197-204; discussion 1204-5. [Crossref] [PubMed]
- Kim SY, Choi M, Hwang HK, et al. Intraoperative Transfusion is Independently Associated with a Worse Prognosis in Resected Pancreatic Cancer-a Retrospective Cohort Analysis. J Clin Med 2020;9:689. [Crossref] [PubMed]
- Ross A, Mohammed S, Vanburen G, et al. An assessment of the necessity of transfusion during pancreatoduodenectomy. Surgery 2013;154:504-11. [Crossref] [PubMed]
- Seykora TF, Ecker BL, McMillan MT, et al. The Beneficial Effects of Minimizing Blood Loss in Pancreatoduodenectomy. Ann Surg 2019;270:147-57. [Crossref] [PubMed]
- Snyder RA, Prakash LR, Nogueras-Gonzalez GM, et al. Perioperative blood transfusions for vein resection during pancreaticoduodenectomy for pancreatic adenocarcinoma: Identification of clinical targets for optimization. HPB (Oxford) 2019;21:841-8. [Crossref] [PubMed]
Cite this article as: Javed AA, Ronnekleiv-Kelly SM, Hasanain A, Pflüger MJ, Habib JR, Wright MJ, He J, Cameron JL, Wolfgang CL, Frank SM, Weiss MJ, Burkhart RA. Blood transfusion is associated with worse outcomes following pancreatic resection for pancreatic adenocarcinoma. Ann Pancreat Cancer 2022;5:1.


