Transformation of metastatic nonfunctioning pancreatic neuroendocrine tumor into insulinoma—two case reports
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are rare neoplasms that originate from endocrine cells within the pancreas. The incidence of PanNETs is less than 1 in 100,000 individuals per year (1). PanNETs are clinically categorized as functioning or nonfunctioning based on hormonal symptoms (2). These hormones include insulin, gastrin, glucagon, vasoactive intestinal peptide, and somatostatin. Approximately 60–90% of PanNETs are nonfunctional (3,4). Nonfunctional PanNETs can rarely transform into functional hormone-secreting PanNETs, which can have life-threatening effects (5-10).
Insulinomas are a type of PanNET with an incidence of 4 cases per million per year (11). Insulinomas are typically benign and cured by resection. However, approximately 10% have malignant characteristics, which means local invasion or distant metastasis. Malignant insulinomas have higher levels of pro-insulin, insulin, C-peptide and are associated with worse survival compared to benign insulinomas (12). Malignant insulinomas tend to have increased relative expression of proinsulin compared to insulin (12). The transformation of a previously nonfunctional PanNET in a malignant insulinoma is exceedingly rare. Due to the paucity of cases in the literature, the management is not well defined. In this report, we describe two rare cases of transformation of metastatic non-functional PanNET into a metastatic functional insulinoma. We present the following cases in accordance with the CARE reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-22-1/rc).
Case description
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent were obtained from the patients’ next of kin for publication of these case reports and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
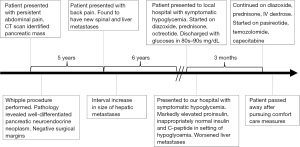
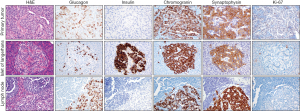
The patient was a 67-year-old male who was initially evaluated after a chief complaint of persistent abdominal pain. Computed tomography (CT) scan found a 2.7 cm × 3.0 cm × 3.8 cm solid, enhancing mass in the pancreatic head without ductal dilatation and small peripancreatic lymph nodes measuring up to 1.4 cm anterior to the pancreatic head. Pancreaticoduodenectomy, partial gastrectomy, and cholecystectomy were performed. Pathology revealed 3.5 cm well-differentiated pancreatic neuroendocrine neoplasm. Immunohistochemical (IHC) labeling revealed that neoplastic cells were for positive synaptophysin and chromogranin, with the Ki-67 proliferation index less than 3%. Neoplastic cells were also negative for insulin but focally positive for glucagon (Figure 1). Negative margins were obtained with 14 of 28 lymph nodes positive for metastatic tumor.
Five years later, the patient developed back pain and was found to have osseous metastases to T11-L4 vertebral bodies, L1 spinous process, and posterior iliac bones bilaterally. He was subsequently incidentally found to have multiple arterial-enhancing hypervascular liver lesions consistent with metastases. Imaging eight and ten years after surgery demonstrated progressive interval increase in size and number of numerous small hepatic metastatic masses. The patient underwent palliative radiotherapy to T11-L2 (2,000 centigray) 7 years after surgery, base of skull and C1-T4 (3,000 centigray) 10 years after surgery, and L4—sacrum (3,000 centigray) 11 years after surgery. Chemotherapy to address progression of disease was delayed due to patient preference.
Eleven years after surgery the patient was admitted to his local hospital for symptomatic hypoglycemia after developing a seizure and loss of consciousness. He was found to have a glucose of 13 mg/dL. During a prolonged 1-month admission, his treatment included intravenous (IV) dextrose solution, diazoxide 100 mg 4 times per day, prednisone 10 mg twice per day, and subcutaneous octreotide 100 mcg three times per day. He transiently had blood glucoses in the 80–90 mg/dL range and was discharged.
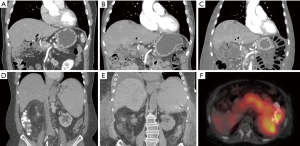
Shortly after, the patient presented to his primary oncologist’s office with a glucose level of 19 mg/dL and was admitted to our hospital. His initial physical exam was significant for pitting edema of the bilateral lower extremities with alert and oriented mental status after glucose repletion. Relevant laboratory studies drawn during episode of hypoglycemia include glucose of 19 mg/dL, proinsulin 352.9 pmol/L (normal range in setting of hypoglycemia: ≥5 pmol/L), insulin 12.5 mcU/mL (normal range in setting of hypoglycemia: ≥3 mcU/mL), and C-peptide 3.81 ng/mL (normal range in setting of hypoglycemia: ≥0.2 ng/mL), and negative insulin autoantibody. Diagnosis of insulinoma was made based on inappropriately elevated insulin, proinsulin, and C-peptide in the setting of hypoglycemia, known hepatic PanNET metastases, and clinical history of prolonged symptomatic hypoglycemia improved with intravenous dextrose. Figure 2 shows a CT scan of his chest, abdomen, and pelvis, which revealed significantly worsened liver and osseous metastatic disease burden compared to prior imaging (Figure 2A-2C).
Treatment for hypoglycemia was directed at metabolically blunting insulin secretion, using cytotoxic agents against the insulinoma, and supplementing with high concentration dextrose solution. The patient was continued on diazoxide, prednisone, and started pasireotide, a newly developed potent somatostatin analog with increased affinity for somatostatin receptor 5 (SSTR5) (13), to decrease insulin secretion. He also had a cytotoxic chemotherapy trial of temozolomide and capecitabine to target the functional tumor, which was based on previous data in metastatic PanNET (14).
Despite these aggressive measures, the patient could not be safely weaned off intravenous dextrose, and he and family decided to pursue comfort measures. The patient died 3 months after initial evidence of tumor functionality. An abbreviated timeline of events is shown in Figure 3.
Case 2
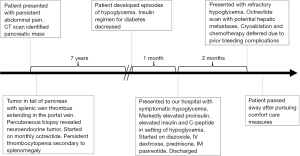
The patient was a 69-year-old male with a history of diabetes mellitus, hypothyroidism, and benign prostatic hyperplasia who was initially evaluated after a chief complaint of abdominal pain. CT scan identified a 6.3 cm × 3.9 cm hyper-enhancing mass in the tail of pancreas obstructing the splenic vein, a thrombus in the splenic vein extending into the portal vein, and splenomegaly. The tumor was not amenable to surgical resection. Percutaneous biopsy was performed, and pathology revealed that neoplastic cells were positive for synaptophysin and chromogranin, with the Ki-67 proliferation index of ~1%, and consistent with neuroendocrine tumor. The patient was started on intramuscular monthly octreotide 30 mg and had persistent thrombocytopenia due to splenomegaly. He had two episodes of upper gastrointestinal bleeding in 4 years after diagnosis, and esophagogastroduodenoscopy showed significant portal hypertension. He was started on propranolol and had no further bleeding complications for several years.
Seven years after diagnosis, the patient developed hypoglycemia. He was previously on insulin for diabetes mellitus, and his insulin regimen was decreased and eventually discontinued over several months. His hypoglycemia became worse, and the patient required increased interventions until he was admitted that year with severe symptomatic hypoglycemia. He was started on diazoxide 250 mg every 8 h then transferred to our hospital for further management.
Relevant laboratory studies drawn during episode of hypoglycemia include glucose 50 mg/dL, proinsulin 1,831.3 pmol/L (normal range in setting of hypoglycemia: ≥5 pmol/L), insulin 26.0 (normal range in setting of hypoglycemia: ≥3 mcU/mL), and C-peptide 6.03 (normal range in setting of hypoglycemia: ≥0.2 ng/mL), negative sulfonylurea screen, and negative insulin autoantibody. Diagnosis of insulinoma was made based on inappropriately elevated proinsulin, insulin, and C-peptide in the setting of hypoglycemia, and the clinical history of prolonged symptomatic hypoglycemia improved with intravenous dextrose. The patient was continued intravenous dextrose, but diazoxide was stopped due to significant fluid retention. He was started on prednisone and long-acting intramuscular pasireotide 40 mg monthly. Chemotherapy was deferred in the setting of persistent thrombocytopenia.
Though the patient was able to be discharged 1 month after initial presentation off intravenous dextrose, he presented 2 months later with symptomatic and refractory hypoglycemia to prednisone 10 mg twice per day and pasireotide 40 mg monthly. There were new hepatic metastases noted on CT scan (Figure 2D-2E). An octreotide scan was performed to assess if there were a focal lesion within the PanNET that was amenable to cryoablation. The octreotide scan found increased uptake in the pancreatic tail and hepatic involvement that was concerning for potential metastatic involvement (Figure 2F). Cryoablation was not pursued due to multiple episodes of gastrointestinal bleeding during this hospitalization. Inpatient chemotherapy with temozolomide and capecitabine was deferred due to bleeding complications. The patient and family decided to pursue comfort measures, and he died 13 months after initial evidence of tumor functionality. An abbreviated timeline of events is shown in Figure 4.
Discussion
Pathophysiology of transformation into functional PanNET
These cases illustrate the rare transformation of a previously nonfunctional neuroendocrine tumor into a functional insulinoma. The exact mechanism of transformation of nonfunctional PanNETs into a functional PanNET is unknown. In terms of genetics and compared to insulinomas, non-functional PanNETs have mutations in alpha-thalassemia/mental retardation X-linked (ATRX) and death domain-associated protein (DAXX) and mammalian target of rapamycin pathway (mTOR) genes (15,16). Insulinomas have unique mutations in epigenetic regulators including Yin Yang 1 (YY1), H3 histone family 3A (H3F3A), lysine-specific demethylase 6A (KDM6A), and ATR serine/threonine kinase (ATR) (15-17). Given the unique mutations of epigenetic modifiers in insulinomas, we hypothesize that non-functional PanNETs with mutations in epigenetic modifiers would be at higher risk for transformation. However, there have been no studies to our knowledge looking at the mutational profile of non-functional PanNETs which have transformed into functional PanNETs. Another related mechanism of transformation may be due to the inherent hormonal plasticity of the neoplastic cells (18). Other studies have also put forth a possible role played by the hepatic microenvironment in transformation of previously nonfunctional PanNETs since all reports had liver metastases at the time of presentation, though the exact mechanism remains unknown (5,6,9,10,19). Repeat biopsy was not considered in the two cases discussed in the present study given the clinical presentation with massively elevated pro-insulin and repeated episodes of hypoglycemia in the setting of known metastatic PanNET. Clinical practitioners should be aware of the possibility of gain-of-function transformation at any point in patients with metastatic PanNETs.
Treatment of metastatic insulinoma
This case report also highlights the difficulties in management of metastatic insulinoma, particularly those with high metastatic burden to the liver. Malignant insulinomas are less responsive to therapies including diazoxide compared to benign insulinomas and require a multimodal approach (20). Medical therapies directed at hypoglycemia include diazoxide, somatostatin analogs (octreotide, lanreotide, pasireotide), and everolimus help to decrease insulin secretion (21).
Diazoxide opens ATP-sensitive potassium channels of pancreatic beta cells, which decreases insulin secretion (22). It is also a potent vasodilator and can result in significant peripheral edema and hypotension.
Somatostatin analogs can have a mixed effect on hypoglycemia, depending on the somatostatin receptors primarily expressed on the insulinoma cells (23). There have been reports of octreotide worsening hypoglycemia in insulinoma, which is thought to be secondary to increased relative suppression of glucagon compared to insulin (24,25). This paradoxical effect is thought to be worse in patients with insulinomas which lack SSTR-2 and SSTR-5 (26). Somatostatin analogs also have potent anti-proliferative effects.
Everolimus is an mTOR inhibitor that has demonstrated improved progression-free survival compared to placebo in patients with advanced PanNET (27). One notable side effect of everolimus is hyperglycemia, and everolimus can be used as a treatment for insulinoma (28,29). Mechanistically, everolimus can be effective against insulinomas by increasing peripheral insulin resistance, decreasing pro-insulin secretion, and reducing cell proliferation (30). Notable adverse effects include pneumonitis and cardiac toxicities (29).
In terms of cytotoxic chemotherapy, streptozocin in combination with doxorubicin and/or fluorouracil have historically been the first-line choice for metastatic PanNET (31-33). Capecitabine and temozolomide regimens are now preferred regimen due to better convenience of oral therapy and favorable side effect profile compared to other regimens (14).
Sunitinib is a tyrosine kinase inhibitor that improves progression-free survival for patients with advanced PanNETs by reducing tumor proliferation and has shown some efficacy as a salvage therapy in a patient with insulinoma (34,35). However, sunitinib is associated with hypoglycemia due to effects on altering glucose metabolism (6,36).
Hepatic artery embolization or chemoembolization can provide significant biochemical and clinical response and is often used palliatively (37,38). Radioembolization utilizing radioactive isotopes can also be considered in refractory cases, though its effects are likely transient (39). Peptide receptor radioligand therapy with Yttrium-90 Dotatoc or Lutetium Lu-177 dotatate are additional options for patients who progress on somatostatin analog therapy (40,41).
Some of the additional treatment modalities mentioned in this section were considered for our patients, but overall clinical stability and goals of care precluded usage. The treatment modalities discussed for metastatic insulinoma have variable clinical efficacy and often have a short-lived therapeutic response. While there have been reports of significant response to certain therapeutics such as everolimus (42), there is no single optimal therapy and our preferred approach is the multimodal one discussed above.
Conclusions
Transformation of a previously nonfunctional PanNET into a functional PanNET is an extremely rare occurrence but is a clinically significant phenomenon that practitioners should be aware of especially in patients with advanced PanNET. While the exact mechanism for this transformation is unknown, the inherent hormonal plasticity of endocrine cells and the hepatic microenvironment where metastases tend to occur may induce transformation of nonfunctional PanNETs into functional tumors. Metastatic insulinoma is difficult to manage, and a multi-pronged approach targeting insulin secretion and malignant cell proliferation should be used. Further research is needed to understand the mechanism of transformation and develop more effective treatments for metastatic insulinoma.
Acknowledgments
We thank the patients’ families for their consent for publication.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-22-1/rc
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-22-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-22-1/coif). RHH and DAL serve as unpaid editorial board members of Annals of Pancreatic Cancer from March 2017 to December 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent were obtained from the patients’ next of kin for publication of these case reports and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589-97. [Crossref] [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [Crossref] [PubMed]
- Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. [Crossref] [PubMed]
- Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012;95:120-34. [Crossref] [PubMed]
- Nahmias A, Grozinsky-Glasberg S, Salmon A, et al. Pancreatic neuroendocrine tumors with transformation to insulinoma: an unusual presentation of a rare disease. Endocrinol Diabetes Metab Case Rep 2015;2015:150032. [Crossref] [PubMed]
- Ohn JH, Kim YG, Lee SH, et al. Transformation of nonfunctioning pancreatic neuroendocrine carcinoma cells into insulin producing cells after treatment with sunitinib. Endocrinol Metab (Seoul) 2013;28:149-52. [Crossref] [PubMed]
- Crona J, Norlén O, Antonodimitrakis P, et al. Multiple and Secondary Hormone Secretion in Patients With Metastatic Pancreatic Neuroendocrine Tumours. J Clin Endocrinol Metab 2016;101:445-52. [Crossref] [PubMed]
- de Mestier L, Hentic O, Cros J, et al. Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: a case-series study. Ann Intern Med 2015;162:682-9. [Crossref] [PubMed]
- Vashi PG, Gupta D, Dahlk S. A unique case of a nonfunctional metastatic pancreatic neuroendocrine tumor transforming into an insulin-secreting tumor with an unusual clinical course. Pancreas 2011;40:781-4. [Crossref] [PubMed]
- Kessoku T, Kobayashi N, Yoneda M, et al. Case Reports: Transformation of End-Stage Neuroendocrine Tumors With Uncontrollable Liver Metastasis Into a Novel or Additional Functional Phenotype. Front Oncol 2020;10:555963. [Crossref] [PubMed]
- Service FJ, McMahon MM, O'Brien PC, et al. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [Crossref] [PubMed]
- Sada A, Yamashita TS, Glasgow AE, et al. Comparison of benign and malignant insulinoma. Am J Surg 2021;221:437-47. [Crossref] [PubMed]
- Siddiqui M, Vora A, Ali S, et al. Pasireotide: A Novel Treatment for Tumor-Induced Hypoglycemia Due to Insulinoma and Non-Islet Cell Tumor Hypoglycemia. J Endocr Soc 2021;5:bvaa171.
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75. [Crossref] [PubMed]
- Hong X, Qiao S, Li F, et al. Whole-genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: leading to a new classification system. Gut 2020;69:877-87. [Crossref] [PubMed]
- Chatani PD, Agarwal SK, Sadowski SM. Molecular Signatures and Their Clinical Utility in Pancreatic Neuroendocrine Tumors. Front Endocrinol (Lausanne) 2020;11:575620. [Crossref] [PubMed]
- Wang H, Bender A, Wang P, et al. Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas. Nat Commun 2017;8:767. [Crossref] [PubMed]
- Chera S, Baronnier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014;514:503-7. [Crossref] [PubMed]
- Arslan MS, Ozbek M, Karakose M, et al. Transformation of nonfunctioning pancreatic tumor into malignant insulinoma after 3 years: an uncommon clinical course of insulinoma. Arch Endocrinol Metab 2015;59:270-2. [Crossref] [PubMed]
- Yasuda A, Seki T, Kitajima N, et al. A case of insulinoma effectively treated with low-dose diazoxide. Clin Case Rep 2020;8:1884-9. [Crossref] [PubMed]
- Hirshberg B, Cochran C, Skarulis MC, et al. Malignant insulinoma: spectrum of unusual clinical features. Cancer 2005;104:264-72. [Crossref] [PubMed]
- Huang Q, Bu S, Yu Y, et al. Diazoxide prevents diabetes through inhibiting pancreatic beta-cells from apoptosis via Bcl-2/Bax rate and p38-beta mitogen-activated protein kinase. Endocrinology 2007;148:81-91. [Crossref] [PubMed]
- Caliri M, Verdiani V, Mannucci E, et al. A case of malignant insulinoma responsive to somatostatin analogs treatment. BMC Endocr Disord 2018;18:98. [Crossref] [PubMed]
- Healy ML, Dawson SJ, Murray RM, et al. Severe hypoglycaemia after long-acting octreotide in a patient with an unrecognized malignant insulinoma. Intern Med J 2007;37:406-9. [Crossref] [PubMed]
- Stehouwer CD, Lems WF, Fischer HR, et al. Aggravation of hypoglycemia in insulinoma patients by the long-acting somatostatin analogue octreotide (Sandostatin). Acta Endocrinol (Copenh) 1989;121:34-40. [Crossref] [PubMed]
- Warner RR. Enteroendocrine tumors other than carcinoid: a review of clinically significant advances. Gastroenterology 2005;128:1668-84. [Crossref] [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [Crossref] [PubMed]
- Kulke MH, Bergsland EK, Yao JC. Glycemic control in patients with insulinoma treated with everolimus. N Engl J Med 2009;360:195-7. [Crossref] [PubMed]
- Bernard V, Lombard-Bohas C, Taquet MC, et al. Efficacy of everolimus in patients with metastatic insulinoma and refractory hypoglycemia. Eur J Endocrinol 2013;168:665-74. [Crossref] [PubMed]
- Fiebrich HB, Siemerink EJ, Brouwers AH, et al. Everolimus induces rapid plasma glucose normalization in insulinoma patients by effects on tumor as well as normal tissues. Oncologist 2011;16:783-7. [Crossref] [PubMed]
- Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71. [Crossref] [PubMed]
- Dilz LM, Denecke T, Steffen IG, et al. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur J Cancer 2015;51:1253-62. [Crossref] [PubMed]
- Krug S, Boch M, Daniel H, et al. Streptozocin-Based Chemotherapy in Patients with Advanced Neuroendocrine Neoplasms--Predictive and Prognostic Markers for Treatment Stratification. PLoS One 2015;10:e0143822. [Crossref] [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Chen J, Wang C, Han J, et al. Therapeutic effect of sunitinib malate and its influence on blood glucose concentrations in a patient with metastatic insulinoma. Expert Rev Anticancer Ther 2013;13:737-43. [Crossref] [PubMed]
- Fountas A, Tigas S, Giotaki Z, et al. Severe resistant hypoglycemia in a patient with a pancreatic neuroendocrine tumor on sunitinib treatment. Hormones (Athens) 2015;14:438-41. [PubMed]
- Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer 2005;104:1590-602. [Crossref] [PubMed]
- Kitano M, Davidson GW, Shirley LA, et al. Transarterial Chemoembolization for Metastatic Neuroendocrine Tumors With Massive Hepatic Tumor Burden: Is the Benefit Worth the Risk? Ann Surg Oncol 2016;23:4008-15. [Crossref] [PubMed]
- Chandra P, Yarandi SS, Khazai N, et al. Management of intractable hypoglycemia with Yttirum-90 radioembolization in a patient with malignant insulinoma. Am J Med Sci 2010;340:414-7. [Crossref] [PubMed]
- Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue 90Y-DOTA-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Bozkirli E, Bakiner O, Abali H, et al. A case of inoperable malignant insulinoma with resistant hypoglycemia who experienced the most significant clinical improvement with everolimus. Case Rep Endocrinol 2013;2013:636175. [Crossref] [PubMed]
Cite this article as: Wu LW, Qasim Hussaini SM, Lee JW, Shu DH, Hruban RH, Laheru DA. Transformation of metastatic nonfunctioning pancreatic neuroendocrine tumor into insulinoma—two case reports. Ann Pancreat Cancer 2022;5:6.