
Patients’ characteristics, diagnosis, and management of pancreatic masses in low resource settings
Introduction
Pancreatic cancer is a global health problem, being the 14th most common cancer and the 7th highest cause of cancer mortality in the world in 2018 (1). While pancreatic cancer is currently most common in high-income countries, its prevalence in low- and middle-income countries is expected to rise. Likewise, the highest mortality rate is estimated to be in Africa, followed by Latin America and the Caribbean, while the lowest incidence will be registered in Europe between 2018 and 2040 (2). Despite this threat, little is known about the disease burden and the presentation of such patients in Tanzania. This calls for a need to increase the identification of these patients, including adopting diagnostic approaches and developing treatment pathways for these patients. The diagnostic investigations for pancreatic masses include both imaging and histology (3). Once a mass is seen, a percutaneous or endoscopic biopsy should be done for all unresectable pancreatic masses, while patients with operable masses should get histology post resection of the mass (4). Additionally, a chest computed tomography (CT) scan or chest X-ray is also indicated to rule out metastasis. These investigations help to properly stage the patients, determine resectability, and discriminate benign from malignant diseases in an attempt to offer the right treatment (5). While the capacity to offer this approach to workup is available, we are unsure how individual patients were handled at our facility. This study seeks to explore potential areas for addressing improvements and identify challenges that will need to be addressed.
Surgery remains the mainstay for the potential cure of pancreatic masses (6). However, it is only feasible in resectable stages, which include American Joint Committee on Cancer (AJCC) stages I and II, and the subset of stage III that is defined as borderline resectable (5). The surgical options for curative surgery are pancreaticoduodenectomy, distal pancreatectomy, and total pancreatectomy depending on the anatomical location of the mass (7). Moreover, even in patients with unresectable disease, there is still a role for surgery to palliate jaundice and gastric outlet obstruction caused by the mass (4-8). To date, the proportion of patients undergoing curative resection in our resource-limited country is not known.
In an attempt to improve outcomes, a better understanding of the disease by both health practitioners and society at large is mandatory. This study gives an insight into the burden of pancreatic mass in Tanzania. It enumerates the clinical characteristics and presentations of patients with a pancreatic mass in Tanzania, shedding light on how these patients are investigated and managed and focusing on areas for improvement to improve the outcomes for these patients. This study demonstrates that now that there is a capacity to offer pancreatic resections, navigating patients to have maximum benefit from care is vital. We present the following article following the STROBE reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-22-3/rc).
Methods
Research context and design
We conducted a hospital-based retrospective cross-sectional study involving patients managed with pancreatic masses at Muhimbili National Hospital (MNH) from January 2018 to December 2019. Data collection was carried out in March 2020. The hospital is located in Dar es Salaam, the commercial capital of Tanzania, and is the most specialized hospital suitable for patients with pancreatic mass. It has one surgical oncologist with an orientation to the gastrointestinal system, four gastrointestinal surgeons, and eight general surgeons, all managing patients with obstructive jaundice (OJ) with or without a pancreatic mass. Also present at the facility is advanced anesthesia care, including a functioning Intensive care unit for post-operative recovery; a functioning endoscopy suite for diagnostic and intervention capabilities; a functioning radiology unit with modern CT scanning and magnetic resonance imaging (MRI), not to mention the basics like X-ray and ultrasonography capacity; and a new interventional radiology unit. The diagnostic laboratory runs tumor markers and offers standard histopathology services. With limited diagnostic and surgical services in the whole country, almost all pancreatic mass patients are handled by this hospital. Patients with pancreatic masses presenting with OJ frequently receive single-by-pass as cholecystojejunostomy (CJ) or double-by-pass as CJ plus gastrojejunostomy.
Study population and sample size
All information from patients with any documented intra-abdominal mass was reviewed for inclusion. From these, all patients with documentation of a pancreatic mass were selected. Patients were included if there was radiological documentation of a pancreatic mass by CT scan or ultrasonography of the abdomen or following clinical exploration for any indication. Patients were of any age and sex and were admitted to the adult surgical units of the hospital.
Measurements
Patients’ age was calculated from the year of birth as years, while sex was taken from the recorded gender and dichotomized as male or female. The area of residence was the administrative region within Tanzania of the original domicile of the patient before coming for medical care, as found in the records. Clinical stage was obtained by reviewing abdominal CT scan (hospital radiological information is stored in Clear Canvas where it was easy to retrieve CT scan and X-rays) and reported according to the World Health Organization (WHO) reporting for pancreatic masses as resectable, borderline resectable, unresectable, or metastatic. Histology was obtained from a patient’s electronic management system report signed by a pathologist. The clinical symptoms and treatment offered were extracted from the case notes as documented by the treating physicians at the time of the original treatment, which are stored in papers in the hospital medical records department. Abdominal ultrasound reports and blood workups, including tumor markers, are available in the patients’ electronic management system.
Data gathering
All patients’ registration numbers and names were retrieved from MNH. Data collection took place in 4 stages. In the first stage, a search was conducted in the hospital’s electronic medical records system to identify and prepare a list of all patients with intra-abdominal masses and OJ. From here, those with pancreatic masses were identified and their file numbers and names were extracted. In the second stage, we searched through the surgical wards’ admission books for patients with a diagnosis of intra-abdominal masses and OJ and made a list with hospital registration numbers and names. The two lists were compared side by side in an excel spreadsheet to identify duplicates and a final list was made. The final list was sent to the medical records for identification of the individual case notes. Two extractors were trained using predefined spreadsheets, which were compared for similarity by the main investigator, NE Kivuyo. Discrepancies were resolved by NE Kivuyo reviewing the case notes and making the final abstraction. From the hospital, the radiology management system, clear canvas, CT scan, and X-ray images of patients who had undergone investigations were reviewed and LO Akoko and one senior radiographer reported the images through consensus.
Data analysis
Data were checked for completeness, de-identified to keep anonymity, coded, and entered into Statistical Package for Social Scientists (SPSS) software version 25 for analysis. Continuous variables were summarized as means with standard deviation, while categorical variables were summarized as means. Missing data were excluded from the item during analysis.
Ethics clearance
The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the Muhimbili University of Health and Allied Sciences (MUHAS) Institutional Review Board (No. MUHAS-REC-09-2020-368) and individual consent for this retrospective analysis were waived. No direct patient identifiers were used during data analysis following the de-identification process.
Results
Study participants’ profiles
In this study, 147 case notes of patients with pancreatic masses were reviewed and their characteristics are depicted in Table 1. The mean age of patients was 60.1±13.6 (range, 27–89) years, with a male to female ratio of 1.16:1. The majority of patients (55.1%) were educated at the primary level, and most of the patients were either peasant (29.9%) or retired (26.5%). The average duration of symptoms before diagnosis was 4 months, with the most common symptom being abdominal pain, which was reported by 72.1% of the patients, followed by jaundice, weight loss, and gastric outlet obstruction, respectively.
Table 1
| Variables | Frequency (%) or mean ± SD [range] (n=147) |
|---|---|
| Age (years) | 60.1±13.6 [27–89] |
| <40 | 16 (10.9) |
| 40–59 | 41 (27.9) |
| 60–69 | 51 (34.7) |
| 70–79 | 32 (21.8) |
| >79 | 7 (4.8) |
| Sex | |
| Male | 79 (53.7) |
| Female | 68 (46.3) |
| Level of education | |
| No formal education | 17 (11.6) |
| Primary level | 81 (55.1) |
| Secondary level | 38 (25.9) |
| Tertiary level | 11 (7.5) |
| Occupation | |
| Peasant | 44 (29.9) |
| Employed | 36 (24.5) |
| Unemployed | 28 (19.0) |
| Retired | 39 (26.5) |
| Presenting symptoms | |
| Abdominal pain | 106 (72.1) |
| Jaundice | 100 (68.0) |
| Weight loss | 82 (55.8) |
| Gastric outlet obstruction | 55 (37.4) |
| Duration of symptoms (months) | 4.72±5.9 [1–36] |
SD, standard deviation.
Risk factors
In Figure 1 below, we present the findings of risk factors collected from patients as documented in their case notes. Cigarette smoking was assessed in all the patients, of which 43 (29.3%) were smokers, all of them being male patients. A total of 141 of the patients were assessed, and 60 (42.6%) of them were taking alcohol, of which men contributed 83.3%. A family history of pancreatic masses was available for 77 of the patients, and only three (3.9%) were reported to have had a family member with a pancreatic mass diagnosis: two of them were male. Of 131 patients with diabetic information recorded, 58 (44.3%) had a diagnosis of diabetes mellitus (DM), with 55% being male patients.
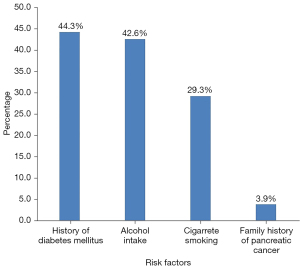
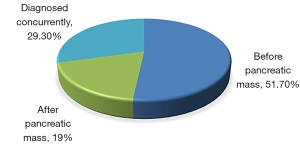
Figure 2 depicts the relationship between the diagnosis of diabetes and the appearance or detection of a pancreatic mass. It was noted that all patients had developed DM in their adult life. In 51.7% of the patients, DM was diagnosed before the pancreatic mass, while in 19% it was diagnosed after the detection of the pancreatic mass, and in 29.3%, the two diseases were diagnosed concurrently.
Diagnosis
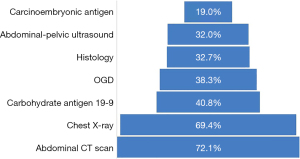
Figure 3 below shows diagnostic procedures that were done on patients to make a diagnosis and stage the patients. Eight investigations were done in varied proportions for these patients, with abdominal CT being the most commonly done, as was in 72.1% of the patients, followed by chest X-ray in 69.4% and the rest as shown. Of significance to note was that only 32.7% had a histological diagnosis and 40.8% had carbohydrate antigen 19-9 (CA19-9).
Histology findings, mass location, and WHO staging
Table 2 below shows that among the 48/147 patients with histological diagnosis, adenocarcinoma was the most common histology (68.8%), followed by inflammation (27.1%), and the rest had a papillary cyst. Most pancreatic masses were located at the head of the pancreas (81.1%) and the least was masses involving the whole pancreas (4.5%). In the WHO staging, it was revealed that only 13.7% of the masses were resectable, whereas the majority (57.3%) presented as metastatic disease. Twenty-three (15.6%) patients were not staged due to the absence of sufficient investigations. Almost a quarter of patients died during their admission before they could receive either palliative or curative treatment.
Table 2
| Variables | Frequency (%) |
|---|---|
| Histology (n=48) | |
| Adenocarcinoma | 33 (68.8) |
| Inflammation | 13 (27.1) |
| Papillary cyst | 2 (4.2) |
| Location of the mass (n=111) | |
| Head | 90 (81.1) |
| Body | 9 (8.1) |
| Tail | 7 (6.3) |
| Whole pancreas | 5 (4.5) |
| WHO staging (n=124) | |
| Resectable | 17 (13.7) |
| Borderline resectable | 11 (8.9) |
| Locally advanced unresectable | 25 (20.2) |
| Metastatic | 71 (57.3) |
| Treatment | |
| Curative surgery | 9 (6.1) |
| Pancreaticoduodenectomy | 5 (55.6) |
| Distal Pancreatectomy | 4 (44.4) |
| Palliative surgery | 83 (56.5) |
| CJ | 30 (36.1) |
| GJ | 23 (27.7) |
| CJ and GJ | 29 (34.9) |
| Only biopsy taken | 1 (1.2) |
| Palliative chemotherapy | 23 (15.6) |
| Died before treatment | 32 (21.8) |
WHO, World Health Organization; MNH, Muhimbili National Hospital; CJ, cholecystojejunostomy; GJ, gastrojejunostomy.
Discussion
This is the first study in Tanzania that we know of to describe pancreatic masses. A total of 147 patients were managed at MNH during the 2 years under review. Only 9 patients (6.1%) had a curative resection, which is lower than global estimates (8). In this study, only a third of patients had histology done, so discrimination of benign from malignant masses was not feasible. Hence, some parameters may be under or overexpressed due to the differences in presentation and management of these two entities. The findings of this study can be used to make inferences on the situation of pancreatic masses in the country since it was done in the National Hospital, which receives patients from all regions of the country.
With several authors reporting that less than 10% of patients are diagnosed under the age of 55, with the majority being diagnosed in their seventh and eighth decades of life (9), it is possible that we have a transition into younger population with different risk factors. But histological diagnosis was only available for one third of the study subjects hence in the majority there is a possibility of observing a different picture. We need to ensure that we understand the complete epidemiology of patients with pancreatic malignancy in our setting by ensuring all patients get a histological diagnosis. Likewise, there was a slight gender predilection, showing a male predominance. The male-to-female ratio was 1.16:1, in line with international and regional studies (10). The reason for this might be greater exposure to some risk factors such as smoking and alcohol, which are expected to be more common among males.
Delay in developing symptoms is a common observation among patients with pancreatic masses, with symptoms developing late in the course of the illness. It is expected that patients will show up for medical consultation right after developing symptoms. In our study, patients took over 4 months from initial presentation to the time of diagnosis. This is a long time compared to that seen in western countries, where patients are usually diagnosed within the first month of symptom presentation (11). Another study in Nigeria showed that most patients presented within the first 2 months (4). The reasons for the delay might be multifactorial, with the low socio-economic status being one of them. Most of our patients had low education levels and were unemployed, which are surrogate markers of low socio-economic status. Another potential cause of delay that will need to be investigated in these patients might be the use of local herbs and the availability of healthcare services, including awareness of the condition among healthcare providers.
Studies have shown cigarette smoking and alcohol intake to be risk factors for pancreatic masses (12-14). The same is depicted in our study, where a third of patients were cigarette smokers and almost half consumed alcohol. A similar picture is depicted in other African countries like Algeria (15). Regarding the family history of pancreatic cancer, only 3.9% of patients were picked in this study, a number very low compared to findings from another study (16). Similarly, a study done in Nigeria showed none of the patients had a family history of pancreatic cancer (4). This difference could be attributed to poor documentation and small sample sizes, which were limitations, mentioned in the later study and may also apply to our study.
It has been shown that DM is a common presentation among patients with pancreatic masses (17). Our study showed that about half of patients had DM, with more than half having the diagnosis before the pancreatic mass. The relationship between pancreatic cancer and DM has been considered to be like that of an egg and chicken. But with diabetic clinics available in many health facilities across the country, the possibility of screening these patients should be explored to improve the early diagnosis of pancreatic masses.
Even though abdominal ultrasound is an important initial investigation in patients with clinical features suggestive of a pancreatic mass (7), it was only performed in one-third of our patients. We, therefore, urge clinicians to adapt this practice to all suspicious patients as some might be missed if this important step is skipped. We also discovered that nearly one out of every three patients lacked an abdominal CT scan, which is critical in the diagnosis and staging of pancreatic tumors (7). Even though CT scan services are readily available in our center, it is not known why only a fraction of patients had this investigation. During the review of abdominal CT scans, it was observed that all of them were abdominal scans lacking the pancreatic protocol. Failure to follow pancreatic protocol could partially explain the low resection rates seen in this study due to inappropriate staging.
Although a chest CT scan is the most preferred imaging to assess for lung and/or pleural metastasis, a chest X-ray is also an alternative (7), but it was done only in two-thirds of our patients. Despite the usefulness of the marker CA19-9 in the diagnosis and follow-up of patients with pancreatic masses (18), less than half of our patients had it done, emphasizing the magnitude of under investigation in this setting. Under-investigation implies that it assigns the wrong stage to the patient and hence the wrong management. Regarding tumor location, our study shows that 8 in 10 of the pancreatic masses were in the head. This is also shown in other studies where masses in the head were more common, followed by the body and tail, respectively (7,15).
The National Comprehensive Cancer Network (NCCN) guidelines recommend that for patients with potentially resectable pancreatic masses, surgery should be done without prior histology. However, for patients with metastatic or locally advanced unresectable disease, a biopsy is required before initiation of chemotherapy treatment (19). However, since pancreas biopsy in non-operated patients can only be obtained by CT- or ultrasound-guided percutaneous fine needle aspiration cytology or by endoscopic ultrasound (20), this explains why a very small proportion of our patients had histology results. We expect the number to increase soon due to a recently established section of interventional radiology in our center that will enable us to perform more percutaneous pancreatic biopsies. With several authors advocating for the use of endoscopic ultrasound-fine needle aspiration biopsy (EUS-FNAB) over percutaneous approaches due to the lower safety and risk of seeding associated with the latter (20), the importance of establishing EUS services in our center cannot be overstated.
This study also identified missed opportunities for establishing histological diagnoses among patients with pancreatic masses. All patients who underwent resection had reported histology. However, among the 83 patients who had palliative surgical procedures, only 39 patients, equivalent to just less than half, had biopsies taken and reported. Overall, the most predominant histology was that of adenocarcinoma, and this is consistent with other literature (15). The fact that almost one-third of the reported histology was inflammation emphasizes the need for histological diagnosis to differentiate benign from malignant disease and hence offer appropriate treatment. Abandonment of care without proper histological diagnosis is worrisome in our setting and denies patients with non-malignant diseases the appropriate treatment while exposing them to unwarranted chemotherapy for palliation.
The mainstay of treatment in unresectable diseases is palliative chemotherapy and palliation of jaundice and/or gastric outlet obstruction. In the absence of self-expanding metal stents in our center, biliary bypass is the alternative option for palliating jaundice. Hence, most of our patients underwent palliative surgery, the leading being CJ, followed by combined CJ and gastrojejunostomy, and then gastrojejunostomy alone. Further studies are needed to evaluate the outcomes of these procedures.
The role of neoadjuvant chemoradiation, particularly in borderline resectable pancreatic cancers, is not negligible as some studies show that it improves treatment outcomes and increases survival rates. In this study, however, no patients with borderline resectable pancreatic mass received neoadjuvant chemoradiotherapy; instead, they were all treated with palliative surgery or palliative chemotherapy. This denies them a chance of cure in selected patients who could benefit from surgical resection following the mentioned therapy.
Conclusions
Pancreatic masses are not a very rare occurrence in our practice. As was expected, patients with pancreatic masses were found to have a late presentation with advanced disease. DM was not uncommon among these patients, either preceding it or diagnosed concurrently with it. There is a lack of a uniform workup of these patients, hence the potential to assign the wrong stage and wrong treatment assignment. This resulted in low resection rates with unclear selection criteria given that some patients with the resectable disease were not offered the choice. Similarly, a few patients had histology results with benign ones not uncommon. There is an urgent need to standardize care for these patients, including investigations and treatment pathways.
Acknowledgments
We would like to acknowledge faculty members and residents of the Department of Surgery, Muhimbili University of Health and Allied Sciences.
Funding: This study was part of the Masters of Medicine in Surgery and received minimal funding from the Tanzania Ministry of Health, Community Development, Gender, Elders, and Children.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-22-3/rc
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-22-3/dss
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-22-3/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-22-3/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the Muhimbili University of Health and Allied Sciences (MUHAS) Institutional Review Board (No. MUHAS-REC-09-2020-368) and individual consent for this retrospective analysis were waived. No direct patient identifiers were used during data analysis following the de-identification process.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Klein AP. Pancreatic cancer: a growing burden. Lancet Gastroenterol Hepatol 2019;4:895-6. [Crossref] [PubMed]
- Imaoka H, Sasaki M, Hashimoto Y, et al. New Era of Endoscopic Ultrasound-Guided Tissue Acquisition: Next-Generation Sequencing by Endoscopic Ultrasound-Guided Sampling for Pancreatic Cancer. J Clin Med 2019;8:1173. [Crossref] [PubMed]
- Alatise OI, Lawal OO, Ojo OT. Challenges of Pancreatic Cancer Management in a Resource Scarce Setting. East and Central African Journal of Surgery 2010;15:52-8.
- Asombang AW, Madsen R, Simuyandi M, et al. Pancreatic Cancer: Patterns in a Low- to Middle- Income Population, Zambia. Med J Zambia 2017;44:212-7.
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Fisher S, Gao H, Yasui Y, et al. Treatment variation in patients diagnosed with early stage breast cancer in Alberta from 2002 to 2010: a population-based study. BMC Health Serv Res 2015;15:35. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Sellam F, Harir N, Khaled MB, et al. Epidemiology and risk factors for exocrine pancreatic cancer in a Northern African population. J Gastrointest Cancer 2015;46:126-30. [Crossref] [PubMed]
- Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014;74:3381-9. [Crossref] [PubMed]
- Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535-45. [Crossref] [PubMed]
- Wang YT, Gou YW, Jin WW, et al. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer 2016;16:212. [Crossref] [PubMed]
- Samokhvalov AV, Rehm J, Roerecke M. Alcohol Consumption as a Risk Factor for Acute and Chronic Pancreatitis: A Systematic Review and a Series of Meta-analyses. EBioMedicine 2015;2:1996-2002. [Crossref] [PubMed]
- Sellam F, Mrabent NM, Harir N, et al. Pancreatic Cancer in a Northern African Population: A Retrospective Analysis Pancreatic Cancer in a Northern African Population: A Retrospective Analysis Spanning Two Decades. JOP. J Pancreas (Online) 2015;16:444-8.
- Hruban RH, Canto MI, Goggins M, et al. Update on familial pancreatic cancer. Adv Surg 2010;44:293-311. [Crossref] [PubMed]
- Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076-83. [Crossref] [PubMed]
- Mohammed S, Van Buren G 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol 2014;20:9354-60. [PubMed]
- Meslar E. Pancreatic adenocarcinoma. JAAPA 2020;33:50-1. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
Cite this article as: Kivuyo NE, Akoko LO. Patients’ characteristics, diagnosis, and management of pancreatic masses in low resource settings. Ann Pancreat Cancer 2022;5:11.


