
Painful tongue lesion as an initial presentation of metastatic pancreatic ductal adenocarcinoma: a case report and review of the literature
Highlight box
Key findings
• Metastasis of pancreatic cancer to the oral cavity, specifically the tongue, is an incredibly rare occurrence.
• Radiation therapy may be beneficial for tongue metastasis.
What is known and what is new?
• This is a rare presentation of pancreatic cancer in which the patient presented with a tongue lesion and was found to have metastatic pancreatic cancer.
What is the implication, and what should change now?
• Consider a broad differential when presented with a patient with a new oral lesion.
• Consider palliative radiation for oral metastasis as a way to improve oral intake.
Introduction
Pancreatic adenocarcinoma is notoriously difficult to detect at an early stage given the location of the pancreas as a retroperitoneal organ, lack of screening tests, and often non-specific initial symptoms. Some of the most common initial symptoms of pancreatic cancer are fatigue, anorexia, weight-loss, abdominal pain, dark urine, jaundice, nausea, or bloating (1,2). Therefore, by the time of diagnosis, many patients present with advanced stage disease. The most common sites for metastases being liver, followed by the peritoneum, lung and pleura, bones, and adrenal glands (3).
Metastasis of solid cancer to the oral cavity is rare and represents less than 1% of neoplasms in the oral cavity (4). In the case of pancreatic cancer, there are only a few documented cases of metastasis of pancreatic cancer to the head and neck region, more specifically the oropharynx and esophagus (5-9). This is a rare way for pancreatic cancer to metastasize and is thought to occur through hematogenous spread from the lung (10). Another commonly discussed mechanism is spread through the Baston venous plexus, which is thought to occur when there is an absence of pulmonary metastases (11). In this case report, we discuss a rare case of pancreatic metastases to the tongue with the tongue lesion being the initial presenting symptom that led to diagnosis of pancreatic adenocarcinoma. We present this article in accordance with the CARE reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-23-10/rc).
Case presentation
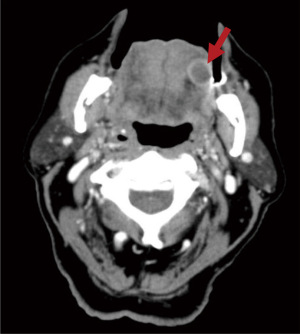
A 91-year-old man who is a former smoker with past medical history of hypertension and hypothyroidism presented to an otolaryngologist [ear, nose and throat (ENT)] office due to a painful left lateral tongue lesion that had been ongoing for approximately 3 weeks. Prior to presentation, his primary care provider (PCP) had prescribed two courses of antibiotics, including amoxicillin and levofloxacin without improvement. His PCP also attempted to drain the lesion with a needle but was unsuccessful given the solid state. He had visited a previous ENT who had obtained a computed tomography (CT) scan showing a 1.5-cm rim-enhancing well-circumscribed lesion in the submucosal left lateral tongue (Figure 1). Physical exam was notable for a firm submucosal lesion on the left lateral tongue with significant tenderness to palpation. Patient underwent an incisional biopsy which showed a moderately differentiated invasive adenocarcinoma without evidence of an associated precursor tumor. The biopsy was positive for cytokeratin 7 (CK7) and caudal-type homeobox 2 (CDX-2), which was suggestive of an upper gastrointestinal (GI) or pancreatobiliary primary, however, a primary salivary gland adenocarcinoma could not be excluded. Patient underwent a positron emission tomography (PET)-CT scan which was notable for a large hypodense lesion within the pancreatic head and tail concerning for a primary pancreatic neoplasm (Figure 2). PET-CT was also notable for metabolically active lesions in the left lateral anterior two-thirds of the tongue, multiple pulmonary nodules, left hilar lymph nodes, soft tissue foci (right shoulder, right lower back, left buttock, and left femur), and a large hypodense right hepatic lesion thought to represent a necrotic hepatic metastasis.

He later presented to the emergency room (ER) with significant tongue pain and poor oral intake resulting in a 20–25-pound weight loss over the past 6 months. Patient was otherwise asymptomatic. Physical exam was notable for the left lateral tongue lesion. Labs were notable for a mild leukocytosis to 13.84×103/µL, elevated alkaline phosphatase to 156 U/L, elevated alanine transaminase to 76 U/L and elevated aspartate aminotransferase to 73 U/L. Patient was treated with supportive measures including fluids and pain medications. Given the unusual location of suspected pancreatic metastasis, patient underwent an endoscopic ultrasound (EUS) with fine needle biopsy (FNB). The procedure showed a 3-cm hypoechoic poorly-demarcated pancreatic tail mass with evidence of extension into the gastric wall with encasement of the splenic vessels, gastric varices, one large 4-cm abnormal lymph node in the portocaval region, and a diffuse abnormal echotexture in the visualized portion of the liver. FNB obtained during the EUS showed invasive adenocarcinoma in the sample taken from the pancreatic tail mass and metastatic adenocarcinoma from the sample taken from the portocaval lymph node. Additional immunostains were performed and compared to the previous tongue biopsy. The tumor from the tongue and from the pancreatic tail lesion were found to be morphologically identical and were consistent with an invasive pancreatic ductal adenocarcinoma (PDAC) with metastasis (Table 1). Somatic testing of the portocaval lymph node specimen demonstrated KRAS G12V gain of function mutation, loss of function mutations in CDKN2A and TP53, copy number gain in PIM1, microsatellite stable, and tumor mutational burden of 4.7 mutations/megabase.
Table 1
| Antibody [clone] | Tongue biopsy result | Pancreatic FNB result |
|---|---|---|
| TTF-1 [SP141] | Negative | – |
| Androgen receptor QL [SP107] | Negative | – |
| Cytokeratin 7 [RN7] | Positive | Positive |
| Cytokeratin 20 [SP33] | Negative | Negative |
| GATA-3 [L50-823] | Negative | – |
| CDX-2 [EPR2764Y] | Positive | Positive |
| DPC4/SMAD4 [B-8] | – | Intact (positive) in tumor cell nuclei |
| Napsin A [1P64] | Negative | – |
| Her2 [pathway] | Negative | – |
| MLH1 [G168-15] | Intact nuclear protein expression | Intact nuclear protein expression |
| PMS2 [A16-4] | Intact nuclear protein expression | Intact nuclear protein expression |
| MSH2 [G219-1129] | Intact nuclear protein expression | Intact nuclear protein expression |
| MSH6 [44] | Intact nuclear protein expression | Intact nuclear protein expression |
FNB, fine needle biopsy; TTF-1, thyroid transcription factor 1; CDX-2, caudal-type homeobox 2.
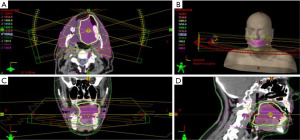
After biopsy confirmed metastatic pancreatic cancer, he was started on gemcitabine monotherapy with plans to add nanoparticle albumin-bound (nab)-paclitaxel with cycle 2 if tolerated. Two weeks after the first dose of gemcitabine, patient developed significant lip swelling and mucositis and was started on dexamethasone with improvement. He was also evaluated by infectious disease and was thought to have herpes of the lip and thrush and was started on acyclovir and fluconazole. The third dose of gemcitabine was held due to grade 3 thrombocytopenia. Additionally, given significant symptoms in his tongue, patient underwent a course of palliative radiation, 1,480 cGy divided in four fractions, quad shot, with improvement in pain and swallowing. Detailed radiation planning imaging is provided in Figure 3 and Figure S1. Moreover, given his poor oral intake, total parenteral nutrition was initiated and later a percutaneous endoscopic gastrostomy tube was placed to supplement nutrition. After two doses of gemcitabine, repeat CT of the abdomen and pelvis showed interval increase in size of the pancreatic mass and progressive hepatic metastatic disease despite chemotherapy. Given the lack of tumor response and poor tolerability to chemotherapy, decision was made to transition to hospice care.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Metastases of pancreatic adenocarcinoma to the tongue is incredibly rare and has only previously occurred in the setting of known pancreatic cancer. To date, there are only two other described cases. Kucuktulu et al. 2013 presented a case of a patient with diagnosed pancreatic adenocarcinoma who had undergone chemotherapy with partial response who later developed a metastasis to the anterior one-third of the tongue (12). The patient’s tongue lesion was treated with radiotherapy and resulted in the mass disappearing macroscopically. de Souza et al. 2016 presented a case of a patient with stage IV pancreatic cancer who developed a lesion on his tongue 60 days into chemotherapy with biopsy proven metastatic pancreatic cancer to the tongue (13). In both cases, these were patients with known pancreatic cancer who later developed lesions of the tongue. This contrasts with our case, where our patient presented with a tongue lesion without a prior diagnosis of pancreatic cancer. Given the rarity of this presentation, it is important to keep a broad differential even in patients with known cancer. In this case, tissue specimens from both the oral lesion and pancreatic lesion confirmed they were morphologically identical. Accurate diagnosis was crucial in prognosticating the patient’s disease and setting an appropriate treatment course.
Adenocarcinomas of the tongue are uncommon, accounting for approximately 2% of all tongue malignancies, and usually follow well-recognized patterns of salivary gland derived carcinomas (14,15). This particular case did not have the appearance of a salivary gland tumor, so a metastasis from another primary tumor was highly suspected. Immunohistochemical stains can quickly identify possible sites of origin and exclude others. Negative thyroid transcription factor 1 (TTF-1) and napsin stain would make a lung primary unlikely (16), and negative GATA-3 and androgen receptor tend to exclude a salivary gland or breast primary (17,18). The combination of CDX-2 and CK7 is common in upper GI tract and pancreatobiliary tumors (19). Negative cytokeratin 20 makes a lower GI tract origin less likely (20). In this case, given the tissue sample was positive for CK7 and CDX-2, this was suggestive of an upper GI or pancreatobiliary primary (Table 1).
Distant spread of pancreatic adenocarcinoma is a poor prognostic sign and often treatments may transition to a more palliative approach depending on a patient’s functional status. According to National Comprehensive Cancer Network (NCCN) guidelines of pancreatic metastatic disease, treatment decisions are based on a patient’s performance status (PS) (21). In those with a good or intermediate PS, first-line therapy is a clinical trial or chemotherapy with FOLFIRINOX (fluorouracil, leucovorin, irinotecan, oxaliplatin) or combination gemcitabine and nab-paclitaxel. However, if patients have disease progression, practitioners can still consider a clinical trial, switching to a different chemotherapy regimen based on tolerability, or supportive care. For patients with poor PS, NCCN guidelines recommend supportive care, single agent chemotherapy, and/or palliative radiation for local disease control.
Radiation therapy (RT) can be used as an adjunct to standard-of-care chemotherapy for metastatic pancreatic adenocarcinoma. According to NCCN guidelines, RT is generally used in five clinical scenarios: resectable/borderline resectable disease, locally advanced disease, in the adjuvant setting after surgical resection, for palliation, or local control for oligometastatic disease recurrence (21). Given symptomatic tongue metastases can greatly impair a patient’s quality of life, RT may be incredibly beneficial from the standpoint of a palliative approach. In the case presented by Kucuktulu et al. 2013, the patient was treated with radiotherapy, specifically a total of 4,320 cGy in 180 cGy fractions with image-guided, intensity-modulated radiation therapy (IG-IMRT) technique in Hi-Art TomoTherapy unit, with significant improvement in the tongue lesion. Similarly, the patient in our case presented with severe pain from his tongue metastasis and it was decided to treat him with a palliative course of “quad shot” radiation. He was treated with 370 cGy twice a day for 2 days with plans for a 3-week break followed by a second course. This regimen is used for quick relief of symptoms and minimizes the risk of mucositis associated with head and neck treatments. He received one round of quad shot RT with good response in the tongue lesion. Given lack of systemic response to chemotherapy, he was ultimately transitioned to hospice and did not undergo the second round of RT. If his response was suboptimal, we would have proceeded with a second cycle of RT and a third cycle could have been considered. Clinicians should consider a course of palliative radiation in these cases to improve symptoms and nutrition, as pain from the lesion could provide a significant barrier to oral intake.
In our case, given the extensive metastatic burden at the time of diagnosis, RT was used with a palliative goal. Given the good clinical response of the tongue metastasis to RT in these two cases, one could consider applications of RT to oligometastatic PDAC. Renz et al. 2017 reviewed data surrounding approaches to oligometastatic disease in pancreatic cancer (22). This study found that for oligometastatic PDAC, specifically with metastasis to the liver, it is not uncommon to use local radio-ablative techniques (22). Studies have shown that use of radiofrequency ablation (RFA) and stereotactic body radiation therapy (SBRT) in PDAC with oligometastatic disease to the liver could be feasible approaches and extend survival in these select patients (23,24).
Radioresistance is a known challenge for treating PDAC and is poorly understood, with many pathways being cited as possible resistance mechanisms (25). Some possible resistance mechanisms include overexpression of HOX transcript antisense intergenic RNA (HOTAIR), which was shown to affect the radiosensitivity of the PDAC cells (26), and manipulation of microRNAs (miRs), specifically miR-31, which could promote a radiosensitive or radioresistant PDAC (27). Given the tongue metastasis had significant clinical response, this appears to be a radiosensitive site of PDAC metastasis. Although it is unknown as to why this would be a radiosensitive site, one could speculate on possible mechanisms. Given research on various resistance mechanisms for PDAC, it is possible that metastasis have a different tumor microenvironment (TME) as compared to the primary tumor that is more favorable for RT. Additionally, studies have pointed to a difficult anatomic location of the pancreas as a contributing factor to radioresistance (26). The anatomical location of the tongue could allow for improved accessibility without as much concern for damaging vital organs (Figure 3 and Figure S1).
In our case, the patient was treated initially with gemcitabine monotherapy with plans to add nab-paclitaxel, however, the disease quickly progressed despite therapy. Gemcitabine is a frequently used treatment option either as monotherapy or in combination based on tolerance (28). However, those on gemcitabine therapy often develop chemoresistance and there have been several postulated mechanisms as to how this happens (29). One of those mechanisms is loss of TP53 function leading to activation of the JAK2-STAT3 pathway. In mouse models, the downstream effects promote alterations in the tumor stroma and TME causing chemoresistance ultimately leading to tumor growth (30). In review of our patients next-generation sequencing (NGS), the tumor demonstrated a frameshift loss of function mutation in TP53, which may have contributed to tumor progression.
Furthermore, the TME of PDAC is characterized by desmoplastic stroma and a host of cells including cancer-associated fibroblasts, stellate cells, tumor-associated macrophages, and other regulatory immune cells that work in concert to promote tumor growth (31). Overexpression of TME regulators on pancreatic cancer cells consequently induces a more immunosuppressive TME and contributes to chemoresistance. Targeting various TME regulators such as focal adhesion kinase (FAK) (32,33) and C-X-C motif chemokine receptor 4 (CXCR4) (34,35) in combination with immunotherapy are active areas of ongoing research and clinical trials (NCT03727880, NCT02907099).
Conclusions
Pancreatic cancer with metastasis to the oral cavity, specifically the tongue, is an incredibly rare presentation. Accurate diagnosis is key to prognosis and appropriate treatment strategies. Given that pancreatic cancer with distant metastases has a poor prognosis, practitioners can consider a more palliative approach when faced with these clinical scenarios. Radiotherapy for symptomatic oral lesions should be highly considered to improve pain and dysphagia that could lead to reduced oral intake.
Acknowledgments
The authors would like to acknowledge the patient and his family for their generosity in allowing us to publish his case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-23-10/rc
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-23-10/coif). AO serves as a section editor (medical oncology) for Annals of Pancreatic Cancer from May 2023 to April 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189-97. [Crossref] [PubMed]
- Holly EA, Chaliha I, Bracci PM, et al. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol 2004;2:510-7. [Crossref] [PubMed]
- Kamisawa T, Isawa T, Koike M, et al. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas 1995;11:345-9. [Crossref] [PubMed]
- Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med 1993;22:385-90. [Crossref] [PubMed]
- Rosati LM, Kummerlowe MN, Poling J, et al. A rare case of esophageal metastasis from pancreatic ductal adenocarcinoma: a case report and literature review. Oncotarget 2017;8:100942-50. [Crossref] [PubMed]
- Burns EA, Kasparian S, Khan U, et al. Pancreatic adenocarcinoma with early esophageal metastasis: A case report and review of literature. World J Clin Oncol 2020;11:83-90. [Crossref] [PubMed]
- Moreira C, Corrales T. Pancreatic adenocarcinoma metastasis to the oral cavity: A rare case report and literature review. Oral Oncol 2021;116:105157. [Crossref] [PubMed]
- Zubović A, Belušić-Gobić M, Harmicar D, et al. Pancreatic Carcinoma Metastatic to the Gingiva. Clin Pract 2021;11:58-64. [Crossref] [PubMed]
- Stecher JA, Mostofi R, True LD, et al. Pancreatic carcinoma metastatic to the mandibular gingiva. J Oral Maxillofac Surg 1985;43:385-90. [Crossref] [PubMed]
- Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, et al. Metastatic tumours to the oral cavity - pathogenesis and analysis of 673 cases. Oral Oncol 2008;44:743-52. [Crossref] [PubMed]
- Batson OV. The function of the vertebral veins and their role in the spread of metastases. 1940. Clin Orthop Relat Res 1995;4-9. [PubMed]
- Kucuktulu E, Kucuktulu U, Guner A, et al. Pancreatic Tumor Metastasis of the Tongue. Journal of Case Reports 2013;3:385-9. [Crossref]
- de Souza BC, Gomes RFT, Schaefer PG, et al. Pancreatic Tumor Metastasis in the Tongue: Case Report. J Interdiscipl Med Dent Sci 2016;4:200. [Crossref]
- Guo C, Jia MQ, Wang L, et al. Primary intestinal-type adenocarcinoma of the tongue. Int J Oral Maxillofac Surg 2018;47:1523-6. [Crossref] [PubMed]
- Murrah VA, Batsakis JG. Salivary duct carcinoma. Ann Otol Rhinol Laryngol 1994;103:244-7. [Crossref] [PubMed]
- Fatima N, Cohen C, Lawson D, et al. TTF-1 and Napsin A double stain: a useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. Cancer Cytopathol 2011;119:127-33. [Crossref] [PubMed]
- Davis DG, Siddiqui MT, Oprea-Ilies G, et al. GATA-3 and FOXA1 expression is useful to differentiate breast carcinoma from other carcinomas. Hum Pathol 2016;47:26-31. [Crossref] [PubMed]
- Kim S, Moon BI, Lim W, et al. Expression patterns of GATA3 and the androgen receptor are strongly correlated in patients with triple-negative breast cancer. Hum Pathol 2016;55:190-5. [Crossref] [PubMed]
- Li MK, Folpe AL. CDX-2, a new marker for adenocarcinoma of gastrointestinal origin. Adv Anat Pathol 2004;11:101-5. [Crossref] [PubMed]
- Kende AI, Carr NJ, Sobin LH. Expression of cytokeratins 7 and 20 in carcinomas of the gastrointestinal tract. Histopathology 2003;42:137-40. [Crossref] [PubMed]
-
Tempero M Malafa M Al-Hawary M. - Renz BW, Boeck S, Roeder F, et al. Oligometastatic Disease in Pancreatic Cancer - How to Proceed? Visc Med 2017;33:36-41. [Crossref] [PubMed]
- Park JB, Kim YH, Kim J, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol 2012;23:635-41. [Crossref] [PubMed]
- Yuan ZY, Meng MB, Liu CL, et al. Stereotactic body radiation therapy using the CyberKnife(®) system for patients with liver metastases. Onco Targets Ther 2014;7:915-23. [Crossref] [PubMed]
- Maity A, Kao GD, Muschel RJ, et al. Potential molecular targets for manipulating the radiation response. Int J Radiat Oncol Biol Phys 1997;37:639-53. [Crossref] [PubMed]
- Jiang Y, Li Z, Zheng S, et al. The long non-coding RNA HOTAIR affects the radiosensitivity of pancreatic ductal adenocarcinoma by regulating the expression of Wnt inhibitory factor 1. Tumour Biol 2016;37:3957-67. [Crossref] [PubMed]
- McGrath J, Kane LE, Maher SG. The Influence of MicroRNA-31 on Oxidative Stress and Radiosensitivity in Pancreatic Ductal Adenocarcinoma. Cells 2022;11:2294. [Crossref] [PubMed]
- Zeng S, Pöttler M, Lan B, et al. Chemoresistance in Pancreatic Cancer. Int J Mol Sci 2019;20:4504. [Crossref] [PubMed]
- Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel) 2017;9:157. [Crossref] [PubMed]
- Wörmann SM, Song L, Ai J, et al. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology 2016;151:180-193.e12. [Crossref] [PubMed]
- Koltai T, Reshkin SJ, Carvalho TMA, et al. Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma: A Physiopathologic and Pharmacologic Review. Cancers (Basel) 2022;14:2486. [Crossref] [PubMed]
- Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851-60. [Crossref] [PubMed]
- Osipov A, Blair AB, Liberto J, et al. Inhibition of focal adhesion kinase enhances antitumor response of radiation therapy in pancreatic cancer through CD8+ T cells. Cancer Biol Med 2021;18:206-14. [Crossref] [PubMed]
- Kato T, Matsuo Y, Ueda G, et al. Enhanced CXCL12/CXCR4 signaling increases tumor progression in radiation-resistant pancreatic cancer. Oncol Rep 2022;47:68. [Crossref] [PubMed]
- Blair AB, Wang J, Davelaar J, et al. Dual Stromal Targeting Sensitizes Pancreatic Adenocarcinoma for Anti-Programmed Cell Death Protein 1 Therapy. Gastroenterology 2022;163:1267-1280.e7. [Crossref] [PubMed]
Cite this article as: Mark C, Minasyan A, Ebia MI, Abbas A, Ghosh A, Watson R, Maluf HM, Hakimian B, Larson B, Mcvay LE, Gong J, Hendifar A, Osipov A. Painful tongue lesion as an initial presentation of metastatic pancreatic ductal adenocarcinoma: a case report and review of the literature. Ann Pancreat Cancer 2023;6:8.


