Modified FOLFIRINOX as the first-line chemotherapy in unresectable and metastatic pancreatic adenocarcinoma—a single-center experience in India
Highlight box
Key findings
• The study evaluated the effectiveness of modified FOLFIRINOX as a first-line chemotherapy regimen for unresectable and metastatic pancreatic adenocarcinoma.
• Among 34 enrolled patients, 52.9% achieved a partial response, and 11.7% had a complete response, resulting in a disease control rate of 88.2%.
• Median progression-free survival was 8 months, and median overall survival was 11 months, indicating improved outcomes compared to historical standards.
What is known and what is new?
• Historically, gemcitabine was the standard treatment, but combination regimens like FOLFIRINOX have shown superiority.
• The study reaffirmed the efficacy of modified FOLFIRINOX, especially in patients with good performance status and minimal comorbidities.
Implications and actions needed
• Modified FOLFIRINOX should be considered viable first-line chemotherapy for suitable patients with this cancer, with patient selection based on performance status, organ function, and comorbidities being vital for optimal outcomes.
Introduction
Pancreatic adenocarcinoma is a devastating disease with a high mortality rate and limited treatment options, particularly in unresectable and metastatic cases. In recent years, significant advancements have been made in the field of first-line chemotherapy for unresectable and metastatic pancreatic adenocarcinoma, offering hope and improved outcomes for patients.
Pancreatic cancer is a rare cancer site, and as per GLOBOCAN 2020 estimate, it ranks 13th in incidence and 7th in mortality worldwide (1). In India, carcinoma pancreas ranks 24th with 12,642 new cases (0.95%) and 19th in mortality (2). As per the SEER 18 2011–2017, most pancreatic cancer is in the advanced stage (52% metastatic vs. 30% regional spread vs. 11% localized, and 7% unknown) with a five-year survival rate of 10.8% (3).
Until 2011, only a few standard options were available for treating pancreatic carcinoma and these were limited to only Gemcitabine when the randomized trial PRODIGE 4/ACCORD 11 demonstrated, a four-drug regimen called FOLFIRINOX prolonged overall survival (OS) compared to gemcitabine monotherapy (11.1 vs. 6.8 months) (4).
Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) study which came in 2013 and showed improved survival with nab-paclitaxel plus gemcitabine combination therapy but with the cost of increased risk of peripheral neuropathy and myelosuppression (5).
Thus, concerns regarding the toxicity profile of standard FOLFIRINOX prompted the evaluation of a modified (attenuated) FOLFIRINOX regimen. A systematic review and meta-analysis included eleven studies and concluded that modified FOLFIRINOX could provide comparative survival benefits with fewer adverse events compared to the conventional dosage (6).
As most of these studies are from other parts of the world and there is a paucity of literature from the Indian subcontinent, we have decided to study the efficacy and toxicity profile of modified FOLFIRINOX-based chemotherapy in locally advanced (unresectable) and metastatic carcinoma pancreas as the first-line chemotherapy in Indian patients.
Methods
It was a prospective observational study. The aim of this study was to evaluate the objective response [complete response (CR)/partial response (PR)/stable disease (SD)/progressive disease], progression-free survival (PFS), and OS of a modified FOLFIRINOX-based chemotherapy regimen in locally advanced (unresectable) and metastatic carcinoma of the pancreas and to evaluate the toxicity profile.
Thirty-four histologically or cytologically proven patients of metastatic pancreatic carcinoma at a tertiary cancer care center in North India were enrolled. Patients were enrolled from November 2019 to June 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Rajiv Gandhi Cancer Institute & Research Centre (RGCIRC), New Delhi (RGCIRC/IRB-BHR/18/2020) and informed consent was obtained from all individual participants.
Inclusion criteria
Eligible adults (≥18 years) with histologically or cytologically proven locally advanced or metastatic adenocarcinoma of the pancreas. Eastern Cooperative Oncology Group (ECOG) performance status (PS) should be 0, 1, and 2. Patients had adequate hematologic, renal & hepatic function (including, a hemoglobin level of ≥9 g/dL, an absolute neutrophil count of ≥1.5×109 per liter, and a bilirubin level at or below the upper limit of the normal range).
Exclusion criteria
The exclusion criteria are as follows: (I) ECOG PS 3, 4; (II) prior chemotherapy will not be permitted; (III) coexistent/synchronous malignancies; (IV) documented brain metastases (brain imaging not required in asymptomatic patients).
Treatment protocol—chemotherapy regimen
Modified FOLFIRINOX regimen including irinotecan 150 mg/m2 day-1, oxaliplatin 85 mg/m2 day-1, leucovorin 400 mg/m2 day-1, 5-fluorouracil (5-FU) 2,400 mg/m2 over 46 hours every 2 weeks for 12 cycles. Dose modification of chemotherapy was permitted according to protocol-specified criteria. Growth factor (PEGylated GCSF) was routinely given to all the patients.
Responses and progression were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 after completion of each four cycles and then based on the response it was decided to plan to continue till twelve cycles of the same chemotherapy then re-evaluation after completion of twelve cycles of chemotherapy, followed by every 3-monthly re-evaluation until disease progression or death.
The primary efficacy endpoints were PFS and OS. Additional efficacy endpoints included the overall response rate (CR + PR) and disease control rate (CR + PR + SD).
Toxicity was assessed at every visit using the National Cancer Institute Common Toxicity Criteria version 5.0. Patients had a complete blood cell count evaluation along with KFT&LFTs before the start of each chemotherapy cycle. If patients had an absolute neutrophil count nadir <500/µL (grade 4 neutropenia), the dose of chemotherapy was reduced by 25% in subsequent cycles. For platelet count nadirs <50,000/µL along with bleeding complications, the dose of chemotherapy in subsequent cycles was reduced by 50%.
Statistical analysis
All analyses were performed with the statistical software SPSS version 23.0. Summary for the continuous variable was presented in mean ± standard deviation or median ± interquartile range (IQR) as per the distribution of the data. Frequency and percentage were presented for categorical variables. For response & progression data, two-sided 95% confidence intervals (CIs) were calculated based on an exact binomial probability at an alpha level of 0.05. Continuous variables were compared using an independent t-test or Mann-Whitney test depending on the normality of the data and categorical variables were compared using the chi-square test or Fisher exact test. Survival analyses were estimated using the Kaplan-Meier method or Cox’s regression model wherever applicable. Statistical significance was defined as P<0.05.
Results
Patients were enrolled from November 2019 to June 2021 at a tertiary cancer care institute in North India and the date of the last follow-up was 31st March 2022. There were 39 histologically proven patients pancreatic adenocarcinoma who were found to be locally advanced (unresectable) or metastatic at presentation registered at RGCIRC in this time period. Among them, two patients were not enrolled due to logistics, three patients did not meet inclusion criteria due to poor performance status. Thirty-four patients received Modified FOLFIRINOX-based combination therapy as a first-line treatment based on inclusion criteria mentioned earlier and were available for final analysis.
In this present study, the majority of cases, 35.30% were in the 61–70 years age group and followed by in the 51–60 years age group. The median age was 56.5 years. The study was performed on 34 patients comprising 25 (73.5%) males and 9 (26.5%) females. The majority (58.8%) of patients were in the locally advanced unresectable stage. Nine patients (26.5%) had liver metastasis, which was the most common site of metastasis. Most of the patients had ECOG PS 1. Head of the pancreas (73.5%) was the most commonly involved primary site. Only 4 (11.8%) patients had normal carbohydrate antigen 19-9 (CA19-9) values and 22 (64.7%) patients underwent biliary decompression prior to starting of systemic chemotherapy. On looking at comorbidities 8 (23.5%) patients were only known cases of diabetes mellitus (DM), 3 (8.8%) were only hypertension (HTN) and 4 (11.8%) had multiple comorbidities. Six (17.6%) patients were smoker and 9 (26.5%) were alcoholics. The baseline clinical characteristics of the entire cohort of 34 patients are comprehensively presented in Table 1.
Table 1
| Patient characteristics | Variables | Value |
|---|---|---|
| Age, years | Mean ± standard deviation | 55.44±8.32 |
| Median [range] | 56.5 [32–70] | |
| Sex | Male | 25 (73.5) |
| Female | 9 (26.5) | |
| ECOG PS at presentation | ECOG PS 0 | 0 |
| ECOG PS 1 | 31 (91.2) | |
| ECOG PS 2 | 3 (8.8) | |
| Stage at presentation | Locally advanced (unresectable) | 20 (58.8) |
| Metastatic | 14 (41.2) | |
| Primary site | Head | 25 (73.5) |
| Body | 5 (14.7) | |
| Tail | 4 (11.8) | |
| Diffuse | 0 | |
| No. of metastatic sites | Non metastatic | 20 (58.8) |
| 1 | 6 (17.6) | |
| 2 | 6 (17.6) | |
| >2 | 2 (5.9) | |
| Liver metastasis | No | 25 (73.5) |
| Yes | 9 (26.5) | |
| CA19-9 levels, U/mL | ≤37 | 4 (11.8) |
| >37 | 30 (88.2) | |
| Previous biliary decompression | No | 12 (35.3) |
| Stent/PTBD | 22 (64.7) | |
| Comorbidities | No | 19 (55.9) |
| HTN | 3 (8.8) | |
| DM | 8 (23.5) | |
| Multiple | 4 (11.8) | |
| Smoker | No | 28 (82.4) |
| Yes | 6 (17.6) | |
| Alcoholic | No | 25 (73.5) |
| Yes | 9 (26.5) |
Data are expressed as n (%) unless otherwise noted. ECOG, Eastern Cooperative Oncology Group; PS, performance status; CA19-9, carbohydrate antigen 19-9; PTBD, percutaneous transhepatic biliary drainage; HTN, hypertension; DM, diabetes mellitus.
A total of 325 cycles of treatment were administered with a median number of 9 chemotherapy cycles administered. Fifteen (44.11%) of patients completed twelve cycles of chemotherapy. The major reason for the discontinuation of chemotherapy was the progression of the disease. One patient had covid pneumonia and died because of same after the 8th cycle and two patients did not receive chemotherapy after 8 cycles because of the surge in covid cases. In one patient oxaliplatin was omitted after the 10th cycle in view of the development of significant neuropathy. Eight patients (23.5%) in this study required a dose reduction at least in one cycle of chemotherapy due to toxicities. Fourteen (41.8%) of patients in this study required delay in at least one cycle of chemotherapy due to grades 3 and 4 toxicities.
Non-hematological toxicities were more common than hematological toxicities. The most common non-hematological toxicities were nausea (55.9%) and fatigue (52.9%). Elevations in liver enzymes were seen in 17.6% of the patients. Additionally, 29.4% of patients suffered from neuropathy, out of which two patients had grade 3 peripheral neuropathy (Table 2).
Table 2
| Adverse events | All grades, n (%) | Grade 3/4, n (%) |
|---|---|---|
| Haematological toxicities | ||
| Neutropenia | 9 (26.5) | 3 (8.8) |
| Leukopenia | 9 (26.5) | 3 (8.8) |
| Anaemia | 14 (41.2) | 2 (5.9) |
| Thrombocytopenia | 10 (29.4) | 3 (8.8) |
| Febrile neutropenia | 2 (5.9) | 0 |
| Non-haematological toxicities | ||
| Vomiting | 13 (38.2) | 0 |
| Nausea | 19 (55.9) | 0 |
| Diarrhoea | 9 (26.5) | 4 (11.8) |
| Stomatitis | 10 (29.4) | 2 (5.9) |
| Fatigue | 18 (52.9) | 1 (2.9) |
| Transaminitis | 6 (17.6) | 2 (5.9) |
| Neuropathy | 10 (29.4) | 2 (5.9) |
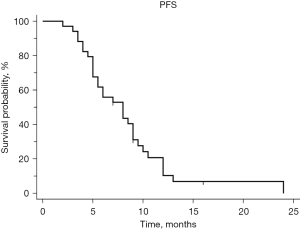
The assessment of the chemotherapy’s effectiveness was conducted through abdominal computed tomography (CT) or magnetic resonance imaging (MRI). In cases where staging was necessary, positron emission tomography-CT was used instead. In total, 52.9% (n=18) of patients had a PR and 11.7% had CR. A total of four (11.7%) patients progressed on chemotherapy and eight (23.53%) had SD. The disease control rate, which includes both responses and SD, was 88.2%. The response rates within different subgroups are summarized in Table 3. The median time to PFS was 8 months (95% CI: 5–9). Six months PFS was 55.9% (Figure 1).
Table 3
| Patient characteristics | Variables | Response, n (%) | P value | |||
|---|---|---|---|---|---|---|
| CR | PR | SD | PD | |||
| Age, years | 31–40 | 0 | 1 (5.56) | 0 | 0 | 0.579 |
| 41–50 | 0 | 8 (44.44) | 1 (12.5) | 1 (25.0) | ||
| 51–60 | 2 (50.0) | 3 (16.67) | 4 (50) | 2 (50.0) | ||
| 61–70 | 2 (50.0) | 6 (33.33) | 3 (37.5) | 1 (25.0) | ||
| ECOG PS at presentation | ECOG PS 1 | 4 (100.0) | 16 (88.89) | 8 (100) | 3 (75.0) | 0.461 |
| ECOG PS 2 | 0 | 2 (11.11) | 0 | 1 (25.0) | ||
| Sex | Male | 4 (100.0) | 13 (72.22) | 5 (62.5) | 3 (75.0) | 0.581 |
| Female | 0 | 5 (27.78) | 3 (37.5) | 1 (25.0) | ||
| No. of metastatic sites | Non metastatic | 2 (50.0) | 10 (55.56) | 6 (75) | 2 (50.0) | 0.814 |
| 1 | 0 | 4 (22.22) | 1 (12.5) | 1 (25.0) | ||
| 2 | 1 (25.0) | 3 (16.67) | 1 (12.5) | 1 (25.0) | ||
| >2 | 1 (25.0) | 1 (5.56) | 0 | 0 | ||
| Liver metastasis | No | 2 (50.0) | 13 (72.22) | 7 (87.5) | 3 (75.0) | 0.581 |
| Yes | 2 (50.0) | 5 (27.78) | 1 (12.5) | 1 (25.0) | ||
| CA19-9 levels, U/mL | ≤37 | 0 | 3 (16.67) | 1 (12.5) | 0 | 0.685 |
| >37 | 4 (100.0) | 15 (83.33) | 7 (87.5) | 4 (100.0) | ||
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; PS, performance status; CA19-9, carbohydrate antigen 19-9.
In the subgroup analysis, the median PFS for patients with locally advanced (LA) disease was 8 months, while for those with metastatic disease it was 6 months. The 6-month PFS rates were 60% for LA and 50% for metastatic disease (P=0.7125). When considering the presence or absence of liver metastases, the median PFS was 8.5 months without liver metastases and six months with liver metastases. The corresponding 6-month PFS rates were 64% and 33.3% respectively (P=0.1799). Among patients with CA19-9 levels below 59 upper limit of normal and above 59 upper limit of normal, the median PFS was 8.5 and 6 months respectively (P=0.1317).
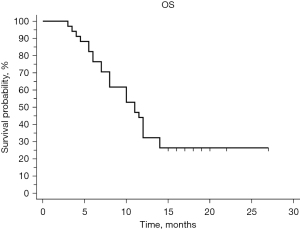
The median OS was 11 months (95% CI: 8–12), and the 6-month OS rate was 76.5% (Figure 2). When comparing LA and metastatic disease, the median OS was 11 months for LA and 10 months for metastatic disease (P=0.6603). For nonmetastatic disease, 1–2 sites, and more than 2 sites, the median OS was 11, 11.5, 10, and 5.5 months respectively (P=0.3750).
Discussion
In recent years, significant advancements have been made in the field of first-line chemotherapy for unresectable and metastatic pancreatic adenocarcinoma, offering hope and improved outcomes for patients.
Historically, gemcitabine was considered the standard first-line chemotherapy for this patient population (7). However, in the past decade, multiple clinical trials have demonstrated the superiority of combination chemotherapy regimens over gemcitabine alone. These regimens have shown increased response rates, prolonged survival, and improved quality of life for patients.
One of the most widely studied and established combination chemotherapy protocols is FOLFIRINOX (8). FOLFIRINOX consists of four drugs: oxaliplatin, irinotecan, leucovorin, and 5-FU. This aggressive regimen, initially introduced in 2010, showed significant efficacy in clinical trials, resulting in improved OS and PFS compared to gemcitabine monotherapy.
The success of FOLFIRINOX, however, comes with an increased risk of toxicity. The regimen is associated with notable adverse effects such as neutropenia, diarrhea, peripheral neuropathy, and fatigue (4). Consequently, patient selection is crucial, and it is generally recommended for individuals with good performance status, preserved organ function, and limited comorbidities. Close monitoring and supportive care measures are essential to manage and mitigate these side effects, ensuring patients receive the optimal benefits of the therapy.
Another notable combination chemotherapy option in the first-line treatment of unresectable and metastatic pancreatic adenocarcinoma is gemcitabine plus nab-paclitaxel (GnP) (5). This combination demonstrated comparable efficacy to FOLFIRINOX in a phase III clinical trial, with a more manageable toxicity profile (9). GnP has become an attractive choice for patients who may not be suitable candidates for FOLFIRINOX due to factors such as age, performance status, or comorbidities.
The success of these combination regimens has revolutionized the management of unresectable and metastatic pancreatic adenocarcinoma. These treatment options have led to improved response rates, increased survival, and better quality of life for patients. It is important to note that individual patient characteristics and preferences should be taken into account when deciding on the most appropriate first-line chemotherapy regimen.
In a retrospective analysis of 100 patients who received modified FOLFIRINOX as their initial treatment, we observed an overall response rate of 52%. This response rate surpassed that of previous studies and reaffirmed the efficacy of this regimen in advanced pancreatic adenocarcinoma. Additionally, the disease control rate, which includes both partial response and stable disease, reached an impressive 80% (10).
In recent years, efforts have also been made to personalize treatment approaches in pancreatic adenocarcinoma based on molecular characteristics of the tumor (11). Biomarkers such as microsatellite instability (MSI) and homologous recombination deficiency (HRD) have been identified as potential predictors of response to specific therapies, such as immune checkpoint inhibitors or poly (ADP-ribose) polymerase (PARP) inhibitors (12). These targeted treatments may offer additional options for selected patients in the first-line setting, particularly those with specific molecular profiles.
Notably, modified FOLFIRINOX demonstrated its greatest impact in terms of PFS and OS. The results of our study, which involved 34 patients, showed a median PFS of 8 months (95% CI: 5–9) and a median OS of 11 months (95% CI: 8–12). Our findings align with some of the previously conducted studies, further contributing to the existing knowledge in this field (Table 4).
Table 4
| Study | Patients | PFS (95% CI) (months) | OS (95% CI) (months) |
|---|---|---|---|
| Our study | 34 | 8 (5–9) | 11 (8–12) |
| Cavanna et al. (13) | 50 | 5.63 (0.4–21.93) | 10.07 (0.53–28.2) |
| Stein et al. (14) | 37 | 6.1 (5.19–8.31) | 10.2 (7.65–14.32) |
| Li et al. (15) | 62 | 7 | 10.3 |
PFS, progression-free survival; OS overall survival; CI, confidence interval.
Another crucial aspect of our experience with modified FOLFIRINOX was the assessment of treatment-related adverse events. While the original FOLFIRINOX regimen was associated with significant toxicity, the modified version offered a more manageable side effect profile. Common adverse events included neutropenia, fatigue, peripheral neuropathy, and gastrointestinal disturbances. However, with appropriate supportive care measures and dose adjustments, the majority of patients were able to tolerate the treatment well.
In our experience, patient selection and careful monitoring played a crucial role in optimizing outcomes with modified FOLFIRINOX. We identified that patients with good performance status, adequate organ function, and minimal comorbidities tended to benefit the most from this regimen. Regular monitoring of blood counts and close attention to potential side effects allowed for timely intervention and dose adjustments, ensuring the treatment’s tolerability.
While our single-center experience with modified FOLFIRINOX has shown promising results, it is important to acknowledge that further prospective, multicenter trials are necessary to confirm these findings and establish its broader applicability. Nonetheless, the outcomes we have observed indicate that modified FOLFIRINOX has the potential to become a standard of care in the first-line treatment of unresectable and metastatic pancreatic adenocarcinoma.
Conclusions
In conclusion, modified FOLFIRINOX has emerged as a viable and effective option for patients with unresectable and metastatic pancreatic adenocarcinoma. Our single-center experience has demonstrated favorable response rates, prolonged PFS, and improved OS. With its manageable toxicity profile and careful patient selection, modified FOLFIRINOX represents a significant advancement in the field of pancreatic cancer treatment. Personalized approaches based on tumor biomarkers also hold promise for further optimizing treatment strategies. Continued research and collaborative efforts are needed to validate these findings and expand access to this promising therapy for a broader population of patients.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-6/dss
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-23-6/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Rajiv Gandhi Cancer Institute & Research Centre (RGCIRC), New Delhi (RGCIRC/IRB-BHR/18/2020) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GLOBOCAN 2020: New Global Cancer Data | UICC [Internet]. [cited 2023 Jun 9]. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data
- An epidemiological review of pancreatic cancer with special reference to India - Indian Journal of Medical Sciences [Internet]. [cited 2023 Jun 9]. Available online: https://ijmsweb.com/an-epidemiological-review-of-pancreatic-cancer-with-special-reference-to-india/
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Tong H, Fan Z, Liu B, et al. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: a systematic review and meta-analysis. Sci Rep 2018;8:8666. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Chan K, Shah K, Lien K, et al. A Bayesian meta-analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PLoS One 2014;9:e108749. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Perri G, Prakash L, Qiao W, et al. Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg 2020;155:832-9. [Crossref] [PubMed]
- Lee MS, Pant S. Personalizing Medicine With Germline and Somatic Sequencing in Advanced Pancreatic Cancer: Current Treatments and Novel Opportunities. Am Soc Clin Oncol Educ Book 2021;41:1-13. [Crossref] [PubMed]
- Singh RR, O’Reilly EM. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs 2020;80:647-69. [Crossref] [PubMed]
- Cavanna L, Stroppa EM, Citterio C, et al. Modified FOLFIRINOX for unresectable locally advanced/metastatic pancreatic cancer. A real-world comparison of an attenuated with a full dose in a single center experience. Onco Targets Ther 2019;12:3077-85. [Crossref] [PubMed]
- Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016;114:737-43. [Crossref] [PubMed]
- Li X, Ma T, Zhang Q, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett 2017;406:22-6. [Crossref] [PubMed]
Cite this article as: Soni S, Jain A, Goyal V, Narayan S, Redhu P, Goyal P, Swamy SS, Talwar V. Modified FOLFIRINOX as the first-line chemotherapy in unresectable and metastatic pancreatic adenocarcinoma—a single-center experience in India. Ann Pancreat Cancer 2023;6:9.