
Quantitative assessment of pancreatic exocrine function in symptomatic patients with locally advanced pancreatic cancer receiving radiation therapy
Highlight box
Key findings
• Quantitative assessment showed that most patients with symptomatic locally advanced pancreatic cancer had decreased exocrine pancreatic function.
What is known and what is new?
• In patients with locally advanced pancreatic cancer, anorexia, nausea, and indigestion are common symptoms.
• Quantitative assessment showed that most patients with symptomatic locally advanced pancreatic cancer had decreased exocrine pancreatic function.
What is the implication, and what should change now?
• Exocrine pancreatic insufficiency is prevalent among patients with locally advanced pancreatic ductal adenocarcinoma. Therefore, pancreas enzyme replacement therapy should be considered as part of treatment, in addition to anti-tumor therapies.
Introduction
The prognosis for locally advanced pancreatic ductal adenocarcinoma (PDAC) is poor, with median overall survival of 19 months (1). Radiation therapy is useful not only to relieve symptoms, but also to improve the effectiveness of treatment (2,3), but treatment-related adverse events such as loss of appetite, nausea, and indigestion should also be considered before radiation therapy.
Bentiromide {PFD® Oral Solution, N-benzoyl-L-tyrosyl-p-aminobenzoic acid, Eisai Co., Ltd., Tokyo, Japan} is a synthetic peptide that is composed of benzoic acid, tyrosine and para-aminobenzoic acid (PABA) (4). It provides a quantitative and non-invasive measure of pancreatic exocrine function. Bentiromide is poorly absorbed orally from the gastrointestinal tract, but is readily and specifically hydrolyzed by α-chymotrypsin, a pancreatic enzyme, to release PABA. PABA is absorbed in the small intestine, conjugated in the liver (mainly glycine conjugation) and excreted in the urine. If the exocrine capacity is impaired in pancreatic disease, bentiromide is not sufficiently degraded and urinary excretion of PABA conjugates is reduced. Therefore, if a given amount of bentiromide is administered orally and the urinary PABA content is measured over a period of time to determine its rate of excretion, this will reflect the exocrine function of the pancreas and allow a quantitative diagnosis of the pancreatic exocrine function (4). Contrary to the usefulness of the PABA test, there are no reports on the quantitative evaluation of pancreatic exocrine function in symptomatic patients with locally advanced PDAC before radiation therapy. The purpose of this study was to evaluate the pancreatic exocrine function quantitatively in symptomatic patients with non-metastatic locally advanced PDAC before radiation therapy.
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Edogawa Hospital (No. RO2017002) and written informed consent was obtained from all patients for use of clinical data in research. This study included a cohort of 24 consecutive patients (12 men and 12 women) who presented with symptomatic manifestations and were diagnosed with unresectable, non-metastatic, locally advanced PDAC prior to radiation therapy within the past two years. The mean age of this cohort of patients was 72.3 years, with a standard deviation (SD) of 11.5 years and an age range of 42 to 86 years. Patients had symptoms of anorexia, nausea, dyspepsia, weight loss greater than 10% in 6 months, pain refractory to opioids, diarrhea, fatty stools or a combination of these. In all cases, pancreatic cancer originated in the head or body of the pancreas. All patients were planned to receive palliative or radical radiation therapy using helical tomotherapy (Accuray, Madison, WI, USA), delivering 30 to 60 Gy in 10 to 30 fractions. We performed the PABA test in patients with PDAC (study arm) and, for comparison, in patients with colorectal carcinoma (CRC) (control arm). The control arm comprised 6 consecutive patients diagnosed with CRC, encompassing 5 male and 1 female individuals. The mean age of the patients was 58.8 years (SD: 7.6 years; range, 53–73 years). These patients in the control arm had previously undergone surgical resection; however, they exhibited the presence of liver metastases or metastases in the upper abdominal lymph nodes within the corresponding time frame. As a result of these conditions, patients in the control arm underwent radiation therapy. In the control arm, chemotherapy was discontinued at least 2 weeks prior to the PABA test. In the study arm, patients with type 1 diabetes mellitus were excluded from the study, but 10 (41.7%) patients had type 2 diabetes mellitus. However, none of patients with CRC (control arm) had pancreatic endocrine insufficiency.
Data collection
In the study arm, patients who had undergone surgery, received pancreatic radiation therapy or chemotherapy, had type 1 diabetes mellitus, severe renal impairment, acute pancreatitis, acute hepatitis, or were receiving pancreatic extract therapy were excluded from the study. Pancreatic exocrine function was assessed quantitatively using the PABA test in both arms. Each patient took 500 mg of bentiromide with at least 200 mL of water after a urine sample on an empty stomach and drank at least 200 mL of water 1 hour after taking bentiromide. All urine samples were collected up to 6 hours after taking bentiromide. Results are expressed as a percentage of the total urinary excretion of PABA up to 6 hours after dosing. Those taking sennoside and acetaminophen stopped taking them 3 days before the PABA test.
Statistical analysis
Results are expressed as mean ± SD with 95% confidence interval (CI), and an unpaired t-test was used to compare the results of patients with PDAC (study arm) and those with CRC (control arm). A P value less than 0.05 was considered statistically significant.
Results
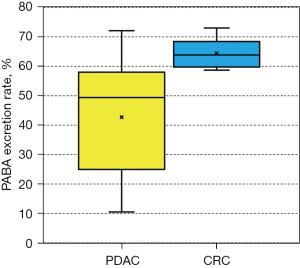
The PABA excretion rate of patients with PDAC (study arm) was 42.7%±18.5% (95% CI: 30.6–54.9%), while the PABA excretion rate of patients with CRC (control arm) was 64.4%±5.1% (95% CI: 60.2–68.6%) (P<0.05) (Figure 1). None (0%) of the 24 patients with PDAC (study arm) had PABA excretion rates in the normal range (normal: 73.4–90.4%), whereas one (16.7%) of the 6 patients with CRC (control arm) had PABA excretion rates in the normal range (P=0.36).
Discussion
The results of this study showed that the rate of PABA excretion is reduced in symptomatic patients with locally advanced PDAC, even in the absence of treatment. This suggests that not only severe malabsorption (5), but also locally advanced PDAC itself may be a risk factor for impaired pancreatic exocrine function. There have been no reports of quantitative assessments of pancreatic exocrine function in patients with unresectable locally advanced PDAC. Patients with locally advanced PDAC experience anorexia, nausea, diarrhea, fatty stools, or indigestion, which may be associated with impaired pancreatic exocrine function. Pancreatic extract therapy (6) may be a viable option to improve the quality of life of such patients.
This study has several strengths. First, this is the first report that symptomatic patients with locally advanced PDAC have impaired exocrine function suggesting the potential usefulness of pancreatic extract therapy. Second, the results of this study suggest that both PDAC-related symptoms and symptoms associated with pancreatic exocrine dysfunction should be considered prior to radiation therapy. By considering both factors and treating pancreatic exocrine dysfunction, the benefits of radiation therapy can be maximized and treatment-related harm can be minimized. The PABA test may be a useful biomarker in patients who cannot undergo stool testing for the assessment of symptoms in patients with locally advanced PDAC undergoing radiation therapy.
There are several limitations to this study. First, the number of patients in the control arm is small, with 24 patients in the study arm and 6 patients in the control arm. Since the purpose of this study was to quantitatively evaluate pancreatic exocrine function prior to radiotherapy for symptomatic locally advanced PDAC, the small number of patients in the control arm is not a limitation. Second, this study does not provide results for radiation therapy or pancreatic extract therapy. Future studies will clarify the effects of radiation therapy or pancreatic extract therapy on pancreatic exocrine function. Third, PABA excretion rates were not compared with standard measures of pancreatic exocrine insufficiency such as fecal elastase 1 and 72-hour fecal fat content. Most patients undergoing radiotherapy have decreased appetite and little or no defecation. We believe that stool testing is not practical for these patients and that urine PABA testing is more practical. The objective of this study was not to compare PABA excretion rates with standard assays, but to quantitatively assess pancreatic exocrine function in symptomatic patients with locally advanced PDAC prior to radiotherapy. Comparison of PABA excretion rates with standard pancreatic exocrine insufficiency tests would be a future research topic. Also, differences in pancreatic exocrine function depending on tumor location are also a topic for future research. Fourth, normal values for PABA excretion rates are based on data from healthy volunteers. The control group consisted of post-operative CRC patients who had already received chemotherapy. Previous treatment and the recurrent tumor itself may have influenced PABA excretion rates in the control group.
Conclusions
Exocrine pancreatic insufficiency is common in patients with locally advanced PDAC and the National Comprehensive Cancer Network (NCCN) recommends pancreatic enzyme replacement therapy. This study provides evidence to support this recommendation and demonstrates that exocrine pancreatic function can be quantitatively assessed in patients who are unable to undergo a stool test.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-12/dss
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-12/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-23-12/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Edogawa Hospital (No. RO2017002) and written informed consent was obtained from all patients for use of clinical data in research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Garnier J, Ewald J, Marchese U, et al. Outcomes of patients with initially locally advanced pancreatic adenocarcinoma who did not benefit from resection: a prospective cohort study. BMC Cancer 2020;20:203. [Crossref] [PubMed]
- Vornhülz M, Anton S, Eross B, et al. Role of stereotactic body radiation in the enhancement of the quality of life in locally advanced pancreatic adenocarcinoma: a systematic review. Radiat Oncol 2022;17:108. [Crossref] [PubMed]
- Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol 2019;14:95. [Crossref] [PubMed]
- Toskes PP. The bentiromide test for pancreatic exocrine insufficiency. Pharmacotherapy 1984;4:74-80. [Crossref] [PubMed]
- Witvliet-van Nierop JE. Exocrine pancreatic and enterocyte function in patients with advanced pancreatic cancer. Clin Nutr 2019;38:2778-82. [Crossref] [PubMed]
- Dhanasekaran R, Toskes PP. Pancrelipase for pancreatic disorders: An update. Drugs Today (Barc) 2010;46:855-66. [Crossref] [PubMed]
Cite this article as: Hama Y, Tate E. Quantitative assessment of pancreatic exocrine function in symptomatic patients with locally advanced pancreatic cancer receiving radiation therapy. Ann Pancreat Cancer 2024;7:2.


