
The impact of KRAS mutations on the clinical outcome and immune response following immunotherapy for pancreatic cancer
Highlight box
Key findings
• KRAS G12D mutant tumors were associated with worse survival outcomes in this resectable pancreatic adenocarcinoma cohort.
• KRAS G12D mutant tumors had lower rates of CD8+ T cell activation following treatment with anti-programmed cell death protein 1 (PD-1) and GVAX treatment.
What is known and what is new?
• KRAS mutations have been increasingly appreciated as mediators of immunosuppression through their influence on the tumor microenvironment and cytokine levels.
• KRAS mutant tumors had higher levels of systemic interleukin 8 than those without mutations in this resectable pancreatic ductal adenocarcinoma (PDAC) cohort.
• This cohort confirmed the inferior survival outcomes with KRAS G12D mutant disease seen in other tumor types.
What is the implication, and what should change now?
• This investigation emphasizes the importance of taking into account KRAS mutation status when treating patients with resectable PDAC.
• The development of KRAS mutation specific inhibitors may offer the opportunity to reverse the unique immunosuppressive features of KRAS mutant tumors.
Introduction
Background
Pancreatic ductal adenocarcinoma (PDAC) is predicted to become the second leading cause of cancer related death in the United States by 2030 with one of the high case-fatality rates of any tumor type (1). Resectable PDAC represents the earliest stage of PDAC and is classically treated with a combination of surgical removal and increasingly intensive chemotherapy (2-4). However despite this aggressive approach, the majority of patients with resectable PDAC will ultimately relapse with systemic disease (4,5). To improve on these stark statistics, the pancreatic cancer multidisciplinary team at Johns Hopkins University has developed a resectable PDAC platform trial in which patients receive two weeks of a novel therapeutic combination prior to surgery followed by additional cycles in the adjuvant setting along with standard of care combination chemotherapy (Figure S1) (6). In Arm A of this platform, we tested our GVAX vaccine, an allogeneic whole cell cancer vaccine given before and after surgery. Correlative analyses on pre- and post-treatment tumors uncovered the induction of lymphoid aggregates and found that the programmed cell death protein 1/programmed cell death protein ligand 1 (PD-1/PD-L1) pathway was upregulated on the T cell and monocyte populations, respectively. This observation inspired Arm B which tested nivolumab (aPD1) in combination with GVAX two weeks before surgery, and continued with the same treatment schedule as in Arm A. Pre-treatment plasma, peripheral blood mononuclear cells (PBMCs), and biopsy specimens were obtained. Surgical specimens along with blood were also collected at the time of surgery.
Rationale and knowledge gap
This trial design has been successful in elucidating the biologic impact of these novel treatment strategies through interrogating surgical resection specimens removed after 2 weeks of neoadjuvant treatment (7,8). Many of the investigations to this point have focused on better understanding the on-target effects of these drugs on the tumor and surrounding tumor microenvironment (TME) but these samples also provide the opportunity to define the effect of clinical and molecular differences on the TME and treatment outcomes.
KRAS mutations present in 90% to 95% of PDACs represent one of the initiating events in tumor development and contribute to biologic aggressiveness through increasing cellular proliferation, inhibiting apoptosis, and angiogenesis (9). In addition to these roles in driving the formation and growth of PDAC, its role in suppressing a productive immune response has become increasingly appreciated. Emerging data has implicated mutant KRAS in increasing the expression of immunosuppressive cytokines to modify circulating and intratumoral immune cell populations. Specifically, KRAS has been implicated in enhancing interleukin 8 (IL-8) production, an immunosuppressive cytokine linked with recruitment of myeloid cells into the TME and conversion to immunosuppression phenotypes (10-12). These elevated circulating IL-8 levels have been previously correlated with worse clinical outcomes with immunotherapy in other tumor types highlighting their clinical importance (11,12). KRAS has also been associated with induction of IL-10 and transforming growth factor-β (TGF-β) which encourages production of a more fibrotic and immunosuppressive TME with conversion of CD4+ T lymphocytes into regulatory T cells (Tregs) (13). In addition to these immunosuppressive cytokine changes, KRAS mutations have been implicated in the downregulation of major histocompatibility complex (MHC) class I molecules limiting the ability of the immune system to detect tumor associated neoantigens (14).
Objective
In this investigation we will explore the influence of KRAS mutations, with a particular focus on KRAS G12D mutant disease, to understand the influence of these molecular alterations on survival outcomes, the TME composition and cytokine levels. We present this article in accordance with the CONSORT reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-24-2/rc).
Methods
IL-8 measurement
De-identified human plasma samples were archived from the NCT02451982 clinical trial (Figure S1). This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The study was approved by the Johns Hopkins Medical Institution Institutional Review Board (IRB)-approved protocol with the approval number IRB00050517 and informed consent was obtained from all individual participants. Plasma samples were collected and stored according to the standard procedure. The concentration of the plasma IL-8 was measured by Quantikine ELISA (R&D Systems, Minneapolis, MN, USA, Cat #: D8000C). In brief, 100 µL of assay diluent RD1-85 was added to each well followed by addition of 50 µL plasma samples or IL-8 standard. The IL-8 standard was prepared according to manufacturer protocol. The plate was then incubated at room temperature for 2 hours followed by four washes using wash buffer supplemented by the ELISA kit. After washing, the plate was inverted and blotted it against clean paper towels to remove residual wash buffer. 100 µL of human IL-8 conjugate was added to each well. The plate was then incubated for 1 hour at room temperature. The washing step was repeated. 200 µL of substrate solution was added to each well with an incubation step for 30 minutes at room temperature in the dark followed by addition of 50 µL of stop solution. The optical density of each well was read using a microplate reader at 450 nM wavelength. A standard curve was generated by using a four-parameter logistic curve fit (GraphPad Prism, Graphpad Boston, MA, USA). The concentration of IL-8 in plasma was measured in duplicate and calculated according to the standard curve.
Multiplex immunohistochemistry (mIHC)
mIHC was performed on resection specimens for patients enrolled on NCT02451982 as part of our previous correlative investigations (6). For this analysis we included the 19 patients treated on this study with GVAX +/− aPD1 that had resection samples that were adequate for mIHC staining/analysis and had at least 2 years of clinical follow up for overall survival. In brief, these tissues samples were obtained 2 weeks after cycle 1 of immunotherapy (6). Formalin-fixed paraffin-embedded (FFPE) tissue blocks from these resection specimens were sectioned into 5-µm thick slides before staining with hematoxylin and scanning using NanoZoomer (Hamamatsu, Shizuoka, JPN). Following antigen retrieval by microwave treatment with the antigen retrieval citra, sequential multiple iterative immunohistochemistry (IHC) cycles involving staining, scanning, and antibody/chromogen stripping, were performed. Negative control images were taken after the last antibody and chromogen stripping. These samples were stained with two immune panels. The first panel designed for CD8+ T-cell markers, included CD45, CD3, CD8, PD-1, CD137, and granzyme B (GZMB). The second staining panel designed for myeloid cell markers and Treg markers, included CD45, CD3, CD8, CD4, CSF-1R, CD68, CD163, CD66b, Foxp3, and TIGIT. Digitized images obtained with NanoZoomer were co-registered via the CellProfiler pipeline (version 2.1.1). Three rectangle regions of interest (ROI)s of ~3,000×3,000 pixels each containing one tumor lymphoid aggregate and epithelial neoplastic cells in the vicinity were chosen for analysis. Immune cell subtypes were defined by their immune cell marker signature as denoted in prior studies. Immune cell density was defined as the percentage of a specific immune cell subtype among all cells within that sample’s tertiary lymphoid aggregates (TLAs). Multiplex IHC analysis was repeated twice with consistent results.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism software (GraphPad) and R version 4.2.3. Disease-free survival and overall survival, starting from the first dose of study treatment, for each patient subgroup were estimated using Kaplan-Meier curves. Survival comparisons were made between patient subgroups using Log-rank tests. Multivariable analysis was performed using Cox proportional hazards model to account for the treatment groups. Comparisons between KRAS mutation status with respect to IL-8 levels and different immune cell densities was performed using a two-tailed Mann-Whitney test for evaluations of two groups and Kruskal-Wallis when comparing three groups. As KRAS G12D was the most commonly identified mutation and has been associated in some cohorts with worse outcomes, this was evaluated as a separate subgroup. The number of samples in each subgroup is listed in the associated figure legends. A P value less than 0.05 was considered statistically significant.
Results
KRAS mutations and survival outcomes
We identified a total of 30 patients from the NCT02451982 clinical trial with known KRAS mutation status and survival outcomes who were treated on Arms A and B of this platform trial. KRAS mutation status was determined using next generation sequencing (NGS). For Arm A, 16 patients were identified, 2 with KRAS wild type disease and 14 with KRAS mutations (G12D: 6, G12R: 2, G12V: 6). For Arm B, 14 patients were identified, 2 with KRAS wild type disease and 12 with KRAS mutations (G12C: 1, G12D: 5, G12R: 2, G12V: 4). Survival for each cohort was estimated using Kaplan Meier survival curves in Figure 1. This demonstrated a worse disease-free survival in the KRAS G12D mutant tumors treated with GVAX alone (P=0.01). Overall survival was also compared between these cohorts revealing a trend towards worse overall survival with KRAS G12D mutant disease in Arm A (P=0.13) and Arm B (P=0.17). We used a multivariable analysis to understand the influence of KRAS G12D status in the combined cohort controlling for treatment arm which identified a significantly worse survival in patients with KRAS 12D disease (P=0.04). We also examined whether KRAS G12D mutations might co-associate with other factors that would influence survival outcomes and/or immune infiltration and impact these associations. We compared age, sex, tumor differentiation (well or moderately differentiated versus poorly differentiated), nodal status (N0 versus N+), stage (1/2 versus 3), TP53 status, CDKN2A status, and SMAD4 status between patients with KRAS G12D mutant PDAC and those without this alteration. For age, we used a student’s t-test to compare these groups and for the other variables we used Fisher’s exact testing. For these variables, there was a trend towards older age (KRAS G12D mutant: mean =70, median =69 years versus not KRAS G12D mutant: mean =65, median =67 years, P=0.11) and increased rate of poorly differentiated tumors in the KRAS G12D mutated cohort (KRAS G12D mutant: 46% versus not KRAS G12D mutant: 15%, P=0.06).
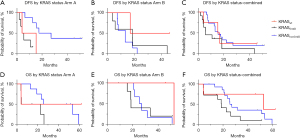
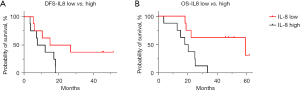
IL-8 levels and KRAS mutation status
Previously reports have suggested IL-8 to be highly immunosuppressive through its recruitment of myeloid cells into the TME and conversion into a more immunosuppressive population. To determine the relationship between IL-8 levels and survival outcomes, baseline plasma IL-8 levels were measured on a total of 17 patients (KRAS G12D: 7, KRAS nonG12D: 8, KRAS wild type: 2). The patients with wild type KRAS PDAC were found to have lower C1D1 IL-8 levels than patients with KRAS mutated disease (P=0.03). To determine the influence of IL-8 levels on survival outcomes, patients were stratified by whether their IL-8 levels were above the median value. Disease-free survival and overall survival for the IL-8 high and IL-8 low groups was estimated via Kaplan-Meier curves. There was a statistically significant (P=0.01) longer overall survival for the group of patients with lower baseline IL-8 levels and a trend (P=0.07) towards longer progression-free survival (Figure 2).
KRAS and TME
KRAS mutations have previously been associated with a number of immunosuppressive functions that would be predicted to significantly alter the TME including IL-8 production. To understand the influence of KRAS mutations on the TME of resectable PDAC, we compared KRAS mutation status with previously obtained mIHC from the PDAC resection specimens. Expressed immunohistochemistry markers were used to identify CD8+ T lymphocytes, CD8+GZMB+ T lymphocytes, CD8+CD137+ T lymphocytes, CD4+ T lymphocytes, regulatory T cells, M1-like macrophages, M2-like macrophages, myeloid derived suppressor cells (MDSCs). Given the profound effects of anti-PD-1 therapy on the TME, arms A and B were analyzed separately for the effects of KRAS mutations. For cohort A, in which patients were treated with GVAX alone, KRAS mutant tumors had a trend towards lower densities of CD8+ T lymphocytes (P=0.07). With the addition of anti-PD-1 treatment to GVAX in Arm B, there was a notably lower percentage of activated CD8+ T lymphocytes as denoted by GZMB+ in the KRAS G12D tumors (P=0.005) (Figure 3). There were no significant association between KRAS mutation status and macrophage or MDSC populations although this may have been limited by small sample size.

Discussion
Key findings
This investigation adds to the growing list of studies reporting on the influence of KRAS mutations on the TME and clinical outcomes. Notably, this investigation identified KRAS mutant resectable PDAC had higher IL-8 levels than KRAS wild type disease which is consistent with prior investigations identifying IL-8 as being upregulated by KRAS mutations (10,14). Higher IL-8 levels were also associated with decreased overall survival in this cohort. These observations raise enthusiasm for therapeutically targeting IL-8 in PDAC and other tumor types. To this end, we have an ongoing investigation evaluating inhibition of IL-8 or its C-X-C chemokine receptor 1/2 (CXCR1/2) receptors in combination with PD-1 blockade which seeks to reverse the deleterious effects of this IL-8 upregulation. Uncovering the TME changes produced by this approach will help inform future strategies combining these agents with other therapies targeting the TME.
The influence of KRAS mutations on the TME was also assessed in resection specimens. This revealed a trend towards reduced CD8+ T lymphocytes in KRAS mutant tumors following treatment with GVAX. As there was only one KRAS G12D tumor in Arm A that had undergone TME assessment this could not be independently assessed. In Arm B, the KRAS G12D mutant tumors had a lower percentage of CD8+ T lymphocytes that were activated as defined by GZMB+. The lack of other significant relationship may have been the result of small sample size or our neoadjuvant treatment approaches. Larger studies would be beneficial in better assessing this question.
Comparison with similar findings
Previous reports have suggested that tumors with KRAS G12D mutations have a particularly poor prognosis with shorter survival following curative intent resection than other KRAS subtypes (15). While the underlying rationale for these inferior outcomes with KRAS G12D mutant disease is poorly understood, prior investigations using cancer cell lines have suggested that KRAS G12D tumors have increased phosphatidylinositol 3-kinase/mitogen-activated protein kinase signaling and less dependency on genes at the G2 and M DNA damage checkpoint than tumors with other KRAS mutations (16,17). Given this observation, we sought to further investigate potential explanations for these outcome differences by assessing IL-8 levels and the TME composition in KRAS G12D mutant PDACs. Looking at the TME, we observed these tumors had a lower percentage of activated CD8+ T lymphocytes as defined by GZMB+ following anti-PD-1 and GVAX treatment. This observation indicates this might be a particularly challenging population to treat with immune checkpoint therapy +/− cancer vaccination, which might be reversed through direct targeting of the KRAS G12D mutation or downstream effectors.
Implications
Targeting mutant KRAS was previously hindered by excess toxicity and rapid development of resistance. More recently KRAS G12C inhibitors have shown promising activity in pancreatic cancer and other G12C mutated tumors (18). While this have also suffered from rapid development of treatment resistance, preclinical studies have identified anti-tumor immune changes including induction of MHCI expression and shift towards a more anti-tumor immune microenvironment with these inhibitors (19). As pan-KRAS inhibitors and KRAS G12D specific inhibitors become available this will become an increasingly attractive opportunity to combine these targeted therapies with immunotherapy to improve treatment durability.
Strengths and limitations
This paper has the strength that it contains a cohort of patients that have undergone neoadjuvant immunotherapy with cancer vaccination +/− anti-PD-1 therapy in the absence of chemotherapy or radiation therapy that might otherwise complicate the TME. This allows for more precise evaluation of the influence of immunotherapy on the TME and the role that KRAS alterations might play in this interaction. This investigation also has several limitations that require consideration when interpreting the results. Most notably this investigation was performed retrospectively on a relatively small number (n=30) of resectable pancreatic cancer specimens with only some of these having plasma for IL-8 level assessment (n=17). These numbers became even smaller when we stratified by KRAS status and treatment group for comparison of the TME limiting our ability to make strong conclusions regarding the influence of KRAS mutation status on TME following neoadjuvant treatments. Another factor is that these samples were from patients enrolled on a clinical trial for resectable PDAC and may not be representative of other PDAC populations including those with metastatic disease or with resectable disease treated in other clinical settings.
Conclusions
In conclusion, this investigation evaluated the influence of KRAS mutations on clinical outcomes, circulating IL-8 levels, and the TME composition in resectable PDAC. This uncovered that KRAS mutated PDACs in this cohort had a trend towards lower CD8+ lymphocyte infiltration following treatment with GVAX. Looking specifically at KRAS G12D tumors, these tumors had a decreased disease-free survival and trend towards a worst overall survival in this cohort compared to patients with KRAS wild type PDAC or other mutations in KRAS and reduced CD8+ lymphocyte activation following GVAX and anti-PD-1 treatment. Further defining the influence of different KRAS mutations on the PDAC TME will help inform future therapeutic strategies integrating inhibition of mutant KRAS or its downstream effectors with immunotherapy to enhance outcomes.
Acknowledgments
We would like to acknowledge the important contribution of our patients, clinical staff, research coordinators, research nurses and regulatory staff to this work.
Funding: This study was partially supported by
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-24-2/rc
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-24-2/dss
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-24-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-24-2/coif). H.W. serves as the unpaid Section Editor (Statistics) of Annals of Pancreatic Cancer from May 2023 to April 2025. LZ serves as the Editor-in-Chief of Annals of Pancreatic Cancer. L.Z. receives grant support from NIH grants (R01 CA169702, R01 CA197296, P50 CA062924), NCI Cancer Center Support Grant P30 CA006973, Biotherapeutics, Bristol-Meyer Squibb, Merck, AstraZeneca, iTeos, Amgen, NovaRock, and Abmeta. L.Z. is a paid consultant/Advisory Board member at Biosion, Alphamab, NovaRock, Ambrx, Akrevia/Xilio, QED, Novagenesis, Snow Lake Capitals, Amberstone, Pfizer, Tavotek, Histosonics, and Mingruizhiyao. L.Z. holds shares at Alphamab, Amberstone, Cellaration, and Mingruizhiyao. E.S.C. has performed consulting for Seres Therapeutics and SIRTeX and has research collaborations with Haystack, Regeneron, Pfizer, Affimed, and NextCure. R.Y. and M.L. are employees of and hold stock in NovaRock. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). The study was approved by the Johns Hopkins Medical Institution Institutional Review Board (IRB)-approved protocol with the approval number IRB00050517 and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilic I, Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J Gastroenterol 2022;28:4698-715. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:439-57. [Crossref] [PubMed]
- Cabasag CJ, Arnold M, Rutherford M, et al. Pancreatic cancer survival by stage and age in seven high-income countries (ICBP SURVMARK-2): a population-based study. Br J Cancer 2022;126:1774-82. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Jones RP, Psarelli EE, Jackson R, et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg 2019;154:1038-48. [Crossref] [PubMed]
- Heumann T, Judkins C, Li K, et al. A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma. Nat Commun 2023;14:3650. [Crossref] [PubMed]
- Li K, Tandurella JA, Gai J, et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell 2022;40:1374-91.e7. [Crossref] [PubMed]
- Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014;2:616-31. [Crossref] [PubMed]
- Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol 2020;21:e135-45. [Crossref] [PubMed]
- Sunaga N, Imai H, Shimizu K, et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int J Cancer 2012;130:1733-44. [Crossref] [PubMed]
- Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 2020;26:688-92. [Crossref] [PubMed]
- Yuen KC, Liu LF, Gupta V, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med 2020;26:693-8. [Crossref] [PubMed]
- Zdanov S, Mandapathil M, Abu Eid R, et al. Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells. Cancer Immunol Res 2016;4:354-65. [Crossref] [PubMed]
- Testorelli C, Bussini S, De Filippi R, et al. Dacarbazine-induced immunogenicity of a murine leukemia is attenuated in cells transfected with mutated K-ras gene. J Exp Clin Cancer Res 1997;16:15-22. [PubMed]
- Shen H, Lundy J, Strickland AH, et al. KRAS G12D Mutation Subtype in Pancreatic Ductal Adenocarcinoma: Does It Influence Prognosis or Stage of Disease at Presentation? Cells 2022;11:3175. [Crossref] [PubMed]
- Cook JH, Melloni GEM, Gulhan DC, et al. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat Commun 2021;12:1808. [Crossref] [PubMed]
- Ihle NT, Byers LA, Kim ES, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst 2012;104:228-39. [Crossref] [PubMed]
- Strickler JH, Satake H, George TJ, et al. Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. N Engl J Med 2023;388:33-43. [Crossref] [PubMed]
- Kemp SB, Cheng N, Markosyan N, et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov 2023;13:298-311. [Crossref] [PubMed]
Cite this article as: Christenson ES, Yu R, Gai J, Wang H, Lei M, Zheng L. The impact of KRAS mutations on the clinical outcome and immune response following immunotherapy for pancreatic cancer. Ann Pancreat Cancer 2024;7:6.


