
Synthesis and evaluation of novel pyrimidine derivative targeting pancreatic adenocarcinoma
Highlight box
Key findings
• The study investigated the antitumor efficacy of pyrimidine derivatives R2 in Ehrlich ascites carcinoma (EAC) and Dalton’s lymphoma ascites (DLA) models. The derivative demonstrated significant inhibition of tumour growth, evidenced by reduced tumour volume and prolonged survival in treated mice compared to controls. R2 exhibited particularly promising results, outperforming the standard drug 5-fluorouracil (5-FU) in both models. Treatment R2 also positively impacted haematological parameters and biochemical profiles, suggesting potential organ protective effects.
What is known and what is new?
• Current knowledge emphasizes chemotherapy’s role in cancer treatment, primarily using agents like 5-FU. This manuscript introduces R2 as potent alternatives with superior efficacy in preclinical models of EAC and DLA. It innovates by demonstrating not only its antitumor effects but also its influence on haematological and biochemical parameters, crucial for overall therapeutic assessment.
What is the implication, and what should change now?
• These findings imply that R2 hold promise as effective therapeutic agent for cancer. Future research should focus on elucidating their mechanisms of action and assessing their translatability to clinical settings. Clinicians and researchers should incorporate these findings into the development of new treatment protocols, especially for cancers where current therapies are insufficient. Regulatory bodies must prioritize evaluating the safety and efficacy of R2 to facilitate its clinical adoption. These actions are essential to potentially enhance cancer treatment outcomes and patient survival.
Introduction
Background
Cancer remains one of the leading global health challenges, characterized by the uncontrolled proliferation of abnormal cells forming malignant tumours with the potential to metastasize. While advancements in treatment modalities have improved outcomes, cancer continues to be a major cause of morbidity and mortality worldwide. Recent studies highlight the complex interplay of genetic, environmental, and lifestyle factors, such as smoking, alcohol consumption, poor diet, and obesity, in the development and progression of cancer (1).
Emerging research has furthered our understanding of cancer’s metabolic alterations. For instance, the Warburg effect—a phenomenon where cancer cells favour glycolysis over oxidative phosphorylation even in the presence of oxygen—has been a focal point in recent studies (2). This metabolic reprogramming provides cancer cells with a survival advantage, contributing to treatment resistance and disease progression. Key enzymes, such as pyruvate dehydrogenase kinases (PDKs), have been identified as crucial regulators in this metabolic shift, particularly in aggressive cancers like pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer. Targeting these enzymes has shown promise in developing new therapeutic strategies (3).
Recent research also underscores the importance of marine-derived compounds in cancer treatment. Studies have identified marine alkaloids and other natural products as potent inhibitors of key cancer-related enzymes, offering a new avenue for drug development. For example, derivatives inspired by marine bis-indolyl alkaloids have demonstrated significant antitumor activity in preclinical models of PDAC, particularly against KRAS-mutant cancer cells, which are notoriously resistant to conventional therapies (4).
These insights underscore the ongoing need for innovative approaches in cancer treatment, leveraging both cutting-edge scientific research and natural product chemistry to overcome the challenges posed by this complex disease.
Objective
Against this backdrop, this research aims to explore the potential of annulated pyrimidine derivatives as novel cytotoxic agents in pancreatic cancer therapy. Leveraging the pharmacological properties of pyrimidine-based compounds, the study seeks to investigate their efficacy and toxicity profiles, with a focus on anticancer activity (5). By elucidating the mechanisms underlying the anticancer properties of annulated pyrimidine derivatives, the research endeavours to contribute to the development of targeted and efficacious treatments for pancreatic cancer, addressing a critical gap in current therapeutic approaches (6).
Rationale and knowledge gap
Within the realm of cancer pathology, pancreatic cancer stands out as a significant contributor to global cancer mortality. Despite advances in oncology, globally, pancreatic cancer ranks 7th in terms of cancer-related mortality, with a staggering number of new cases and fatalities reported annually (7). Notably, regions such as Asia, with burgeoning populations, face a disproportionate increase in pancreatic cancer incidence and mortality, highlighting the urgency for comprehensive research and interventions (8). While efforts have been made to elucidate the aetiology of pancreatic cancer, challenges persist due to its multifactorial nature, with cigarette smoking and familial predisposition emerging as predominant risk factors (9). Moreover, the grim prognosis associated with pancreatic adenocarcinoma underscores the imperative for early detection and innovative therapeutic approaches (10).
Methods
Materials
Drugs and chemicals
4'methoxyacetophenone, 4'nitro acetophenone, 4-(trifluoromethyl) benzaldehyde, guanidine hydrochloride and 2-naphthaldehyde were bought from Sigma Aldrich and 5-fluorouracil (5-FU) was sourced from Central Drug House, New Delhi. On the day of the trials, all other reagents utilized in the study were newly produced and of analytical quality.
Cell lines and cell culture media
Cell lines PaCa-2 and PanC-1 (pancreas cancer cell line) were obtained from The University of Trans-disciplinary Health Sciences and Technology (TDU) located at Yelahanka, Bengaluru, Karnataka 560064.
Cell culture media Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS), Ehrlich ascites carcinoma (EAC) and Dalton’s lymphoma ascites (DLA) cancer cells were obtained from Amla Cancer Research Centre, Thrissur, Kerala 680555.
Experimental animals
A population of 25–30 g, 8–12-week-old, healthy Swiss albino mice were used to screen for anti-cancer activities. The animals were kept in conventional environmental conditions in polypropylene cages with controlled humidity, a 12-hour light/dark cycle, and standard feed pellets and water available at all times. Following the rules of CPCSEA, New Delhi, India, and with permission from Acharya & BM Reddy College of Pharmacy’s Institutional Animal Ethical Committee (IAEC), Bengaluru, the experimental procedures were carried out (reference number: IAEC/ABMRCP/2021-22/22), in compliance with IAEC, Bengaluru national or institutional guidelines for the care and use of animals.
In-silico studies
Docking studies
Docking studies were performed using Discovery Studio 3.5 software. The receptor preparation involved obtaining the three-dimensional configuration of the epidermal growth factor receptor tyrosine kinase (PDB CODE-7KK6) from the Protein Data Bank (PDB). Ligand preparation was conducted using Chem Draw and Discovery Studio 3.5. Molecular docking was carried out using the CDOCKER protocol in Discovery Studio 3.5 (11).
Identification and characterization
Using a variety of techniques, such as mass spectrometry, Fourier-transform infrared (FTIR), Fourier-transform chromatography (TLC), nuclear magnetic resonance (NMR) spectroscopy, and melting point measurement, the produced compounds were identified and described (12-15).
In vitro studies
Cytotoxicity assessment of the compounds was conducted on the MIA PaCa-2 human pancreatic cancer cell line and the PanC-1 human epithelioid pancreatic adenocarcinoma cell line using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Various concentrations (250, 125, 62.5, 31.25, 15.06 µg/mL) of the compounds (R1, R2, R3) and the standard drug 5-FU were tested. These specific concentrations were chosen to encompass a range that includes both sub-lethal and potentially cytotoxic levels, allowing for a comprehensive evaluation of the dose-response relationship. The assay protocol involved seeding cells in 96-well plates, treating them with the compounds, incubating for an appropriate time, adding MTT solution to assess cell viability, solubilizing formazan crystals, and recording absorbance at the designated wavelength (16).
This range was selected to determine the minimum effective concentration as well as the concentration at which cytotoxic effects become pronounced. By spanning a wide range of concentrations, the study aims to identify the half maximal inhibitory concentration (IC50) value with greater accuracy, which is critical for evaluating the therapeutic potential of the compounds in comparison to standard treatments like 5-FU.
In vivo toxicity assessment in mice
Acute oral toxicity studies were performed on female Swiss albino mice according to OECD Test Guidelines 425. The limit test was conducted at 2,000 mg/kg body weight of compound R2, with observations recorded over a period of 14 days (17).
In vivo studies
The anticancer activity of compound R2 was screened against EAC and DLA carcinoma-induced cancer in mice. Dose selection was based on acute oral toxicity studies. Cancer cell count and induction were performed by injecting cancer cells intraperitoneally into donor mice. Treatment protocols involved administering the compounds orally at specified doses for 21 days, followed by evaluation of antitumor, antioxidant, serum estimation, haematological parameters, and histopathology (18,19). The grouping of animals for the EAC/DLA cell line model is detailed in Table 1.
Table 1
| Serial No. | Groups | Treatment (dose and route of administration) | No. of animals |
|---|---|---|---|
| 1 | Normal control | Normal saline (1 mL/100 g, p.o.) | 6 |
| 2 | Positive control | EAC-induced group (i.p.)/DLA-induced group (normal saline, 1 mL/100 g, p.o.) |
8 |
| 3 | Treatment-1 | R2 (200 mg/kg, p.o.) + EAC/DLA induced (i.p.) | 8 |
| 4 | Treatment-2 | R2 (400 mg/kg, p.o.) + EAC/DLA induced (i.p.) | 8 |
| 5 | Standard | 5-FU (20 mg/kg, i.p.) + EAC/DLA induced (i.p.) | 8 |
EAC, Ehrlich ascites carcinoma; DLA, Dalton’s lymphoma ascites; p.o., per oral; i.p., intraperitoneal; 5-FU, 5-fluorouracil.
Parameters
Body weight analysis
Following the inoculation of tumour cells, the mice’s body weight was recorded every week (20). The following calculation was used to get the % reduction in body weight:
where, Gc = gain in the body weight of the control group, Gt = gain in the body weight of the treated group.
Determination of haematological parameters
Blood was withdrawn from each mouse using the retro-orbital plexus method for haematological evaluation, including red blood cell (RBC), white blood cell (WBC), haemoglobin, and platelet count (21).
Tumour volume measurement
Using a graduated centrifuge tube, ascitic fluid was extracted from the peritoneal cavity and its volume was determined (20).
Measurement of mean survival time (MST) and percentage increase in life span (% ILS)
The mortality rate was noted every day until every animal had passed away, and the percentage of ILS was computed using the following formula (22):
Estimation of biochemical parameters and antioxidant assays
After 21 days of treatment, biochemical parameters were evaluated. Blood was drawn from one mouse per group by cardiac puncture for the analysis of serum creatinine kinase, lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) (23). Liver and kidney tissue homogenate was prepared for further biochemical estimations and antioxidant assays.
Estimation of superoxide dismutase (SOD)
Liver tissue supernatant was used to perform spectrophotometric analysis to measure SOD activity. Units of the enzyme activity per milligram of protein were given (24).
Estimation of catalase (CAT)
CAT activity was measured using liver tissue supernatant by UV spectrophotometer. The unit of enzyme activity per milligram of protein was used to express the enzyme activity (24).
Estimation of reduced glutathione (GSH)
GSH content was measured using liver homogenate and DTNB reagent. At 412 nm, the absorbance was measured, and the results were given as nanomoles of GSH per milligram of protein (24).
Histopathology studies
Liver and kidney tissues were fixed in 10% formalin and subjected to histopathological examination after sacrificing the animals (25).
Statistical analysis
The data were analysed using one-way analysis of variance (ANOVA) and the Dunnett test. The data were presented as mean ± standard error of the mean (SEM) (n=6). The statistical significance thresholds were set with Graph Pad Prism 10 (version 10.0.2) at *P<0.05, **P<0.01, and ***P<0.001.
Results
Synthesis of the designed compounds
Synthesis of intermediate 3a [3-(4-methoxyphenyl)-1-(4-(trifluoromethyl) phenyl) prop-2-en-1-one]
4'-methoxy acetophenone (0.75 g, 0.005 mmol) was poured into the RBF. It was then dissolved with 40% ethanol + NaOH (10 mL). 4-(trifluoromethyl) benzaldehyde (0.78 mL, 0.005 mmol) was added to the reaction mixture. Then it was kept for continuous stirring for 6 h by maintaining the temperature of 0–4 ℃ with the help of an ice bath. The completion of the reaction was confirmed by TLC. After confirmation of the completion of reaction, the reaction mixture was poured into ice water. The reaction mixture was then neutralized by dil. HCl. After neutralization the reaction mixture was filtered and dried in shade. Yield 60%, M.P. 55–57 ℃.
Synthesis of intermediate 3b [1-(naphthalen-2-yl)-3-(4-nitrophenyl) prop-2en-1 one]
4'-nitro acetophenone (0.99 g, 0.006 mmol) was poured into the RBF. It was then dissolved with 40% Et. NaOH (10 mL). 2-naphthaldehyde (0.86 mL, 0.006 mmol) was added to the reaction mixture. Then it was kept for continuous stirring for 6 h by maintaining the temperature of 0–4 ℃ with the help of an ice bath. The completion of the reaction was confirmed by TLC. After confirmation of the completion of reaction, the reaction mixture was poured into ice water. The reaction mixture was then neutralized by dil. HCl. After neutralization the reaction mixture was filtered and dried in shade. Yield 55%, M.P. 62–64 ℃.
Synthesis of R1 [4-(4-methoxyphenyl)-6-(4-(trifluoromethyl) phenyl) pyrimidin-2-amine]
Intermediate 3a (0.01 mol), guanidine hydrochloride (0.01 mol, 0.6 g) was dissolved in 40% Et. NaOH and guanidine hydrochloride as added to the reaction mixture. It was then kept for reflux at 70 ℃ for 2–4 hours. TLC was observed in between to ensure that the reaction had finished. Following reaction completion, the mixture was allowed to cool to room temperature before being added to ice cold water and dilute hydrochloric acid was added to neutralize it. After obtaining the precipitate, it was filtered, cleaned with water, and dried. Yield 57%, M.P. 142–148 ℃, 1H NMR (400 MHz), DMSO, (δ ppm) 8.68 (s, 1H, CH-Ar), 8.16–8.14 (d, 2H, CH-Ar), 7.94–7.83 (m, 4H, CH-Ar), 7.577.73 (d, 2H, CH- Ar), 6.89 (s, 2H, NH2), 3.33 (s, 3H, OCH3), [M] 345.15. [M+2] 347.20.
Synthesis of R2 [4-(naphthalen-2-yl)-6-(4-nitrophenyl) pyrimidin-2-amine]
Intermediate 3b (0.01 mol), guanidine hydrochloride (0.01 mol, 0.6 g) was dissolved in 40% Et. NaOH and guanidine hydrochloride as added to the reaction mixture. It was then kept for reflux at 70 ℃ for 2–4 hours. TLC was observed in between to ensure that the reaction had finished. Following reaction completion, the mixture was allowed to cool to room temperature before being added to ice cold water and dilute hydrochloric acid was added to neutralize it. After obtaining the precipitate, it was filtered, cleaned with water, and dried. Yield 49%, M.P. 146–151 ℃, FTIR cm-1 3515 (N-H), 2920 (C-H), 1555 (C=C), 1511,1319 (-NO2), 1H-NMR, (400 MHz), DMSO, (δ ppm) 8.78 (s, 1H, CH-Ar), 8.76–8.66 (d, 2H, CH-Ar), 8.47–8.44 (d, 2H, CH-Ar), 8.41–8.27 (m, 4H, CH-Ar), 6.80 (s, 2H, NH2).
In vitro anticancer activity evaluation by MTT assay
The In vitro anticancer activity for the synthesized novel pyrimidine derivatives was carried out by MTT assay against MIA PaCa-2 and PanC-1 cell lines. The results are visually presented in Figures 1,2, which graphically depict the percentage viability of the derived compounds at different concentrations against MIA PaCa-2 and PanC-1 cells, respectively.


As detailed in Table 2, the IC50 values demonstrate that compound R1 is the most effective against the MIA PaCa-2 cell line, while in PanC-1, compound R2 exhibits the highest cytotoxic activity. These findings underscore the differential efficacy of the synthesized compounds across the two cell lines, providing crucial insights into their potential therapeutic applications.
Table 2
| Serial No. | Sample | IC50 (µg/mL) against MIA PaCa-2 cells | IC50 (µg/mL) against PanC-1 cells |
|---|---|---|---|
| 1 | R1 | 139.7 | 122.54 |
| 2 | R2 | 141.387 | 52.68 |
| 3 | R3 | 247.11 | 360.44 |
| 4 | 5-FU | 123.33 | 2,166.4 |
IC50, half maximal inhibitory concentration; R1, 4-(4-methoxyphenyl)-6-(4-(trifluoromethyl) phenyl) pyrimidin-2-amine; R2, 4-(naphthalen-2-yl)-6-(4-nitrophenyl) pyrimidin-2-amine; R3, 4-cyclohexyl-4''-methoxy-[1,1':3',1''-terphenyl]-5'-amine; 5-FU, 5-fluorouracil.
In vivo anticancer activity
EAC model
Effect on body weight
EAC-induced groups exhibit an increase in body weight, which was monitored weekly over 14 days. As shown in Figure 3, the treatment with novel pyrimidine at two separate doses (200 and 400 mg/kg), resulted in a significant decrease in body weight compared to the EAC-induced group. The data indicates that the 400 mg/kg dose group effectively reduced body weight more significantly than the 200 mg/kg dose group, showcasing a dose-dependent effect. This reduction contrasts with the standard treatment and highlights the potential efficacy of the novel pyrimidine in mitigating weight gain associated with EAC.

Effect on haematological parameters
(I) Effect of novel pyrimidine on RBC count-EAC model
The impact of a novel pyrimidine, administered in two different doses (200 and 400 mg/kg), along with the standard 5-FU (20 mg/kg), on RBC counts in the EAC model is illustrated in Figure 4. The treatment with the novel pyrimidine at both doses (400 and 200 mg/kg), demonstrated significant improvements in RBC levels, with P<0.05 and P<0.001, respectively, compared to the EAC-induced mice. Notably, the 400 mg/kg dose was more effective in increasing RBC counts than the 200 mg/kg dose.

(II) Effect of novel pyrimidine on WBC count-EAC model
The effect of novel pyrimidine in two separate doses 200 and 400 mg/kg and standard 5-FU (20 mg/kg) on WBC in EAC model is given in Figure 4. Treatment with the novel pyrimidine at both 400 and 200 mg/kg doses resulted in a significant decrease in WBC counts (P<0.001) compared to the EAC-induced mice. Notably, the 400 mg/kg dose was more effective in reducing WBC counts than the 200 mg/kg dose.
(III) Effect on hemoglobin (Hb) content-EAC model
The effect of novel pyrimidine in two separate doses, the low dosage (200 mg/kg) and the high dose (400 mg/kg) and standard 5-FU (20 mg/kg) on Hb content in EAC model is given in Figure 4. Treatment with the high dose of novel pyrimidine (400 mg/kg) and the low dose (200 mg/kg) both resulted in a significant increase in Hb content (P<0.001) compared to EAC-induced mice. The high dose (400 mg/kg) led to a greater increase in Hb content than the low dose (200 mg/kg).
(IV) Effect on platelet count-EAC model
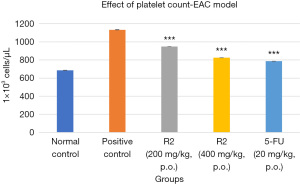
The effect of novel pyrimidine in two separate doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on platelet count in EAC model is illustrated in Figure 5. Both doses of the novel pyrimidine (200 and 400 mg/kg) resulted in a significant decrease in platelet count compared to the EAC-induced mice, with a P<0.001. The 400 mg/kg dose was particularly effective, leading to a lesser platelet count than the 200 mg/kg dose.
Effect of novel pyrimidine on serum enzyme parameters
(I) Effect on serum AST-EAC model
The effect of novel pyrimidine administered at two separate doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on serum AST levels in the EAC model is shown in Figure 6. Treatment with novel pyrimidine at doses of 400 and 200 mg/kg resulted in a significant decrease in AST levels (P<0.001) compared to the EAC-induced mice. The 400 mg/kg dose was more effective in reducing AST levels than the 200 mg/kg dose. The results indicate that both 400 and 200 mg/kg doses of novel pyrimidine significantly decreased AST levels compared to the EAC-induced group, with the 400 mg/kg dose showing a more pronounced effect than the 200 mg/kg dose.

(II) Effect on serum ALT-EAC model
The effect of a novel pyrimidine at two separate doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on serum ALT estimation in the EAC model is shown in Figure 6. Treatment with the novel pyrimidine at 400 and 200 mg/kg resulted in a significant decrease in ALT compared to the EAC-induced mice (P<0.001). The 400 mg/kg group showed a more effective reduction in ALT compared to the 200 mg/kg group. The results indicate a significant decrease in ALT levels in both the 400 and 200 mg/kg groups compared to the EAC-induced group, with the 400 mg/kg group demonstrating a more substantial decrease compared to the 200 mg/kg group.
(III) Effect on serum LDH-EAC model
The effect of a novel pyrimidine at two separate doses (200 and 400 mg/kg) and the standard 5-FU (20 mg/kg) on serum LDH in the EAC model is shown in Figure 6. Treatment with the novel pyrimidine at 400 and 200 mg/kg resulted in a significant decrease in LDH (P<0.001) compared to the EAC-induced mice. The 400 mg/kg group showed a more effective decrease in LDH compared to the 200 mg/kg group. Overall, significant reductions in LDH were observed in both the 400 and 200 mg/kg groups compared to the EAC-induced group, with the 400 mg/kg group showing a more pronounced effect.
(IV) Effect on serum creatine kinase (CK)-EAC model
The effect of the novel pyrimidine at two separate doses (200 and 400 mg/kg) and the standard 5-FU (20 mg/kg) on serum CK in the EAC model is illustrated in Figure 7. Treatment with the novel pyrimidine at both 400 and 200 mg/kg doses showed significant results (P<0.001), with a decrease in CK levels compared to the EAC-induced mice. The high-dose group (400 mg/kg) demonstrated a more pronounced decrease in CK compared to the low-dose group (200 mg/kg). These results indicate a significant reduction in CK levels in both the 400 and 200 mg/kg groups compared to the EAC-induced group, with the 400 mg/kg dose being more effective than the 200 mg/kg dose.

Effect of novel pyrimidine on tumour volume
The effect of the novel pyrimidine at two separate doses (200 and 400 mg/kg) and the standard 5-FU (20 mg/kg) on tumour volume in the EAC model is illustrated in Figure 8. Treatment with the novel pyrimidine at both doses showed significant results (P<0.001), with a reduction in tumour volume compared to the EAC-induced control group. The high-dose group (400 mg/kg) demonstrated a more pronounced decrease in tumour volume compared to the low-dose group (200 mg/kg).

Effect of novel pyrimidine on tissue antioxidant parameters
(I) Effect of novel pyrimidine on tissue antioxidant parameters (SOD, CAT, GSH)
The effects of the treatment on SOD, CAT, and GSH are illustrated in Figure 9. The positive control group exhibited decreased levels of SOD and CAT compared to the normal control group. Treatment with R2 at both 200 and 400 mg/kg doses, as well as 5-FU at 20 mg/kg, resulted in a significant increase (P<0.001) in SOD and CAT levels compared to the positive control group. For GSH, the positive control group showed increased levels compared to the normal control group, while treatment with R2 at both doses and 5-FU significantly decreased GSH levels (P<0.001) compared to the positive control group.

(II) Effect of novel pyrimidine on tissue antioxidant parameters [malondialdehyde (MDA), myeloperoxidase (MPO)]
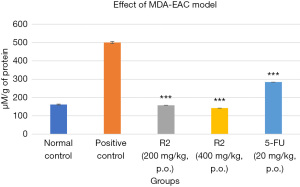
The effects of the treatment on MDA and MPO are illustrated in Figures 10,11, respectively. The positive control group had increased MDA and MPO levels compared to the normal control group. Treatment with R2 at both 200 and 400 mg/kg doses, as well as 5-FU at 20 mg/kg, resulted in a significant decrease (P<0.001) in MDA and MPO levels compared to the positive control group.

DLA model
Effect on body weight-DLA model
DLA-induced groups exhibit an increase in body weight, which was monitored weekly over 14 days. As shown in Figure 12, treatment with novel pyrimidine at two different doses (200 and 400 mg/kg) resulted in a significant decrease in body weight compared to the DLA-induced group. The data indicates that the 400 mg/kg dose group effectively reduced body weight more significantly than the 200 mg/kg dose group, demonstrating a dose-dependent effect. This reduction contrasts with the standard treatment and underscores the potential efficacy of the novel pyrimidine in mitigating weight gain associated with DLA-induced conditions.

Effect on haematological parameters
(I) Effect of novel pyrimidine on RBC count-DLA model
The effect of novel pyrimidine at two different doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on RBC count in the DLA model is shown in Figure 13. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant increase in RBCs compared to the DLA-induced mice (P<0.001). The 400 mg/kg group was more effective in increasing RBCs compared to the 200 mg/kg group.

(II) Effect of novel pyrimidine on WBC count-DLA model
The effect of novel pyrimidine at two different doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on WBC count in the DLA model is shown in Figure 13. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant decrease in WBCs compared to the DLA-induced mice (P<0.001). The 400 mg/kg group was more effective in decreasing WBCs compared to the 200 mg/kg group.
(III) Effect on Hb content-DLA model
The effect of novel pyrimidine at two different doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on Hb content in the DLA model is shown in Figure 13. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant increase in Hb compared to the DLA-induced mice (P<0.001). The 400 mg/kg group was more effective in increasing Hb compared to the 200 mg/kg group.
(IV) Effect on platelet count-DLA model
The effect of novel pyrimidine at two different doses, 200 and 400 mg/kg, as well as the standard 5-FU (20 mg/kg), on platelet count in the DLA model is shown in Figure 13. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant increase in platelet count compared to the DLA-induced mice (P<0.001). The 200 mg/kg group was more effective in increasing platelet count compared to the 400 mg/kg group.
Effect of novel pyrimidine on tumour volume
The effect of the novel pyrimidine at two separate doses (200 and 400 mg/kg) and the standard 5-FU (20 mg/kg) on tumour volume in the DLA model is illustrated in Figure 14. Treatment with the novel pyrimidine at both doses showed significant results (P<0.001), with a reduction in tumour volume compared to the DLA-induced control group. The high-dose group (400 mg/kg) demonstrated a more pronounced decrease in tumour volume compared to the low-dose group (200 mg/kg).

Effect of novel pyrimidine on serum enzyme parameters
(I) Effect on serum AST-DLA model
The effect of novel pyrimidine at two separate doses, 200 and 400 mg/kg, and the standard 5-FU (20 mg/kg) on serum AST in the DLA model is shown in Figure 15. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant decrease (P<0.001) in AST compared to DLA-induced mice. The 400 mg/kg group showed a more pronounced reduction in AST compared to the 200 mg/kg group. These results indicate that both doses of novel pyrimidine significantly decreased AST levels compared to the DLA-induced group, with the 400 mg/kg dose being more effective than the 200 mg/kg dose.

(II) Effect on serum ALT-DLA model
The effect of novel pyrimidine at two separate doses, 200 and 400 mg/kg, and the standard 5-FU (20 mg/kg) on serum ALT in the DLA model is shown in Figure 15. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant decrease (P<0.001) in ALT compared to DLA-induced mice. The 400 mg/kg group demonstrated a more effective reduction in ALT compared to the 200 mg/kg group. These results indicate that both doses of novel pyrimidine significantly decreased ALT levels compared to the DLA-induced group, with the 400 mg/kg dose being more effective than the 200 mg/kg dose.
(III) Effect on serum LDH-DLA model
The effect of novel pyrimidine at two separate doses, 200 mg/kg and 400 mg/kg, and the standard 5-FU (20 mg/kg) on serum LDH in the DLA model is shown in Figure 15. Treatment with novel pyrimidine at 400 and 200 mg/kg resulted in a significant decrease (P<0.001) in LDH compared to DLA-induced mice. The 400 mg/kg group exhibited a more pronounced reduction in LDH compared to the 200 mg/kg group. These results indicate that both doses of novel pyrimidine significantly decreased LDH levels compared to the DLA-induced group, with the 400 mg/kg dose being more effective than the 200 mg/kg dose.
(IV) Effect on serum CK-DLA model
The effect of the novel pyrimidine administered in two separate doses (200 and 400 mg/kg) and the standard 5-FU (20 mg/kg) on serum CK in the DLA model is shown in Figure 16. Treatment with the novel pyrimidine at doses of 400 mg/kg and 200 mg/kg resulted in a significant decrease in CK levels (P<0.001) compared to the DLA-induced mice. The 400 mg/kg dose was particularly effective, leading to a more pronounced reduction in CK levels compared to the 200 mg/kg dose. The results indicate a significant decrease in CK levels for both the 400 and 200 mg/kg treatments compared to the DLA-induced group, with the 400 mg/kg group demonstrating a more effective reduction in CK levels than the 200 mg/kg group.

Effect of novel pyrimidine on tissue antioxidant parameters
(I) Effect of novel pyrimidine on tissue antioxidant parameters (SOD, CAT, GSH)
The effects of the novel pyrimidine on tissue antioxidant parameters (SOD, CAT, GSH) are illustrated in Figure 17. The positive control group exhibited decreased levels of SOD and CAT compared to the normal control group. Treatment with R2 at both 200 and 400 mg/kg doses, as well as 5-FU at 20 mg/kg, resulted in a significant increase (P<0.001) in SOD and CAT levels compared to the positive control group. For GSH, the positive control group showed increased levels compared to the normal control group, while treatment with R2 at both doses and 5-FU significantly decreased GSH levels (P<0.001) compared to the positive control group.

(II) Effect of novel pyrimidine on tissue antioxidant parameters (MDA, MPO)
The effects of the treatment on MDA and MPO levels are illustrated in Figures 18,19, respectively. The positive control group exhibited increased MDA and MPO levels compared to the normal control group. Treatment with R2 at both 200 and 400 mg/kg doses, as well as 5-FU at 20 mg/kg, resulted in a significant decrease (P<0.001) in MDA and MPO levels compared to the positive control group.


Discussion
Molecular docking
Molecular docking is a pivotal bioinformatics tool used to predict the interaction between small molecules and target proteins (26,27). In our study, we employed molecular docking to evaluate the binding affinity of four newly synthesized pyrimidine derivatives, R-1 to R-4, with the PARP receptor, a key protein involved in DNA repair mechanisms. Among these, R1 and R2 exhibited particularly strong binding scores of −8.3 and −8.0, respectively, suggesting favourable interactions. The strong binding affinity of R1 and R2 to the PARP receptor could indicate their potential role as PARP inhibitors, which may sensitize cancer cells to DNA-damaging agents and induce apoptosis through impaired DNA repair mechanisms.
Synthesis of the molecule
The synthesis process involved the creation of intermediate compounds, 3a and 3b, which served as precursors for R1 and R2. These intermediates were synthesized through reactions involving specific reagents under controlled conditions, yielding compounds suitable for subsequent testing.
Cytotoxicity study—in vitro MTT assay
The In vitro MTT assay was employed to assess the cytotoxic effects of R1, R2, and other compounds on MIA PaCa-2 and PanC-1 pancreatic cancer cell lines (28,29). The assay revealed promising results, with R2 demonstrating significant cytotoxicity against both cell lines, surpassing the efficacy of R1, R3, and R4. Notably, R2 exhibited lower IC50 values, indicating its potent inhibitory effect on cancer cell viability compared to the standard drug 5-FU. The cytotoxic effects of R2 may be attributed to its strong binding affinity to the PARP receptor, which could lead to the disruption of DNA repair processes and induce cell death. Additionally, R2’s ability to induce oxidative stress and disrupt cellular redox balance could further contribute to its anticancer effects.
In vivo study
In the in vivo study using EAC and DLA models, the efficacy of R2 was evaluated. Treatment with R2 significantly prolonged the MST of mice compared to the untreated groups. The reduction in ascitic fluid volume and body weight in treated mice suggests that R2 may exert its antitumor effects by inhibiting tumour growth and reducing tumour-induced ascites. The molecular mechanisms underlying R2’s in vivo efficacy may involve the inhibition of angiogenesis and the induction of apoptosis in tumour cells, leading to decreased tumour burden and improved survival outcomes.
Haematological parameters
Treatment with R2 and the standard drug resulted in favourable changes in haematological parameters, including increased haemoglobin levels, red RBC counts, and platelet counts, along with decreased WBC counts. These findings suggest that R2 possesses hematopoietic supportive properties, which are beneficial in cancer treatment scenarios.
Serum estimation
Biochemical analysis revealed that R2 treatment led to a significant reduction in serum markers such as AST, ALT, LDH, and CK, compared to untreated EAC and DLA-induced groups. This indicates the protective effect of R2 on liver and kidney function, crucial in mitigating cancer-induced organ damage.
Antioxidant estimation
Antioxidant assays demonstrated that R2 enhanced the activity of enzymes like SOD and CAT, while maintaining or increasing GSH levels compared to the control groups. These antioxidants play critical roles in combating oxidative stress associated with cancer, highlighting R2’s potential as an antioxidant-rich therapeutic agent.
Conclusions
In conclusion, R2 exhibited promising anti-cancer properties in both in vitro and in vivo models, demonstrating significant cytotoxicity, enhanced survival rates, and beneficial effects on haematological and biochemical parameters. The findings underscore R2 as a potential candidate for further exploration in cancer therapy, owing to its robust molecular interactions, synthesis feasibility, and therapeutic efficacy. Importantly, these findings contribute to the expanding body of knowledge in cancer research, highlighting R2’s potential for clinical application and offering a new avenue for future research. Future studies should focus on elucidating its unique mechanisms of action, optimizing dosage regimens, and exploring its efficacy across different cancer types to fully realize its clinical potential. The novelty of R2’s efficacy suggests it could represent a significant advancement in the development of targeted cancer therapies.
Acknowledgments
The authors express their gratitude to the administration and personnel of Acharya & BM Reddy College of Pharmacy for their unwavering support and encouragement during the research project. Completing this research has been made possible by their guidance and assistance.
Funding: None.
Footnote
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-24-21/dss
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-24-21/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-24-21/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No.: IAEC/ABMRCP/2021-22/22) granted by the institutional ethics board of Acharya & BM Reddy College of Pharmacy, in compliance with Institutional Animal Ethical Committee (IAEC), Bengaluru national or institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Carbone D, De Franco M, Pecoraro C, et al. Structural Manipulations of Marine Natural Products Inspire a New Library of 3-Amino-1,2,4-Triazine PDK Inhibitors Endowed with Antitumor Activity in Pancreatic Ductal Adenocarcinoma. Mar Drugs 2023;21:288. [Crossref] [PubMed]
- Schepis T, De Lucia SS, Pellegrino A, et al. State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers (Basel) 2023;15:3423. [Crossref] [PubMed]
- Carbone D, Pecoraro C, Panzeca G, et al. 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Nortopsentin Derivatives against Pancreatic Ductal Adenocarcinoma: Synthesis, Cytotoxic Activity, and Inhibition of CDK1. Mar Drugs 2023;21:412. [Crossref] [PubMed]
- Singh P, Anand A, Kumar V. Recent developments in biological activities of chalcones: a mini review. Eur J Med Chem 2014;85:758-77. [Crossref] [PubMed]
- Tylińska B, Wiatrak B, Czyżnikowska Ż, et al. Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study. Int J Mol Sci 2021;22:3825. [Crossref] [PubMed]
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis 2010;28:645-56. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 2010;24:417-22. [Crossref] [PubMed]
- Jarag KJ, Pinjari DV, Pandit AB, et al. Synthesis of chalcone (3-(4-fluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one): advantage of sonochemical method over conventional method. Ultrason Sonochem 2011;18:617-23. [Crossref] [PubMed]
- Ustabaş R, Süleymanoğlu N, Özdemir N, et al. New chalcone derivative: synthesis, characterization, computational studies and antioxidant activity. Lett Org Chem 2019;17:46-53. [Crossref]
- Jin C, Liang YJ, He H, et al. Synthesis and antitumor activity of novel chalcone derivatives. Biomed Pharmacother 2013;67:215-7. [Crossref] [PubMed]
- Gopinathan A, Moidu M, Mukundan M, et al. Design, synthesis and biological evaluation of several aromatic substituted chalcones as antimalarial agents. Drug Dev Res 2020;81:1048-56. [Crossref] [PubMed]
- Golan T, Atias D, Barshack I, et al. Ascites-derived pancreatic ductal adenocarcinoma primary cell cultures as a platform for personalised medicine. Br J Cancer 2014;110:2269-76. [Crossref] [PubMed]
- OECD. Test No. 425: Acute oral toxicity: up-and-down procedure. 2022.
- Pak PJ, Kang BH, Park SH, et al. Antitumor effects of herbal mixture extract in the pancreatic adenocarcinoma cell line PANC1. Oncol Rep 2016;36:2875-83. [Crossref] [PubMed]
- Geetha B, Santhy KS. Tumor reducing and anticarcinogenic activity of green tea against Daltons ascites lymphoma cells. Int J Pharma Med Biol Sci 2013;2:92-6.
- Rao L, Yu GT, Meng QF, et al. Cancer cell membrane-coated nanoparticles for personalized therapy in patient-derived xenograft models. Adv Funct Mater 2019;29:1905671. [Crossref]
- Sajid M, Yan C, Li D, et al. Potent anti-cancer activity of Alnus nitida against lung cancer cells; in vitro and in vivo studies. Biomed Pharmacother 2019;110:254-64. [Crossref] [PubMed]
- Ramalingam S, Adithiya RA, Joseph A, et al. Anticancer activity of Ipomoea carnea on Ehrlich ascites carcinoma bearing mice. Indian J Pharm Educ Res 2019;53:703-9. [Crossref]
- Qi S, Li X, Dong Q, et al. Chinese Herbal Medicine (Xiaoaiping) Injections for Chemotherapy-Induced Thrombocytopenia: A Randomized, Controlled, Multicenter Clinical Trial. J Altern Complement Med 2019;25:648-55. [Crossref] [PubMed]
- Ahmed NM, Youns MM, Soltan MK, et al. Design, Synthesis, Molecular Modeling and Antitumor Evaluation of Novel Indolyl-Pyrimidine Derivatives with EGFR Inhibitory Activity. Molecules 2021;26:1838. [Crossref] [PubMed]
- Inamura K. Update on Immunohistochemistry for the Diagnosis of Lung Cancer. Cancers (Basel) 2018;10:72. [Crossref] [PubMed]
- Morris GM, Lim-Wilby M. Molecular docking. Methods Mol Biol 2008;443:365-82. [Crossref] [PubMed]
- Kitchen DB, Decornez H, Furr JR, et al. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 2004;3:935-49. [Crossref] [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63. [Crossref] [PubMed]
- Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107-12. [Crossref] [PubMed]
Cite this article as: Panduranga Mudgal M, Mallapura Vijayakumar M, Tiwari RK, Purawarga Matada GS, Janadri S, Sharma UR. Synthesis and evaluation of novel pyrimidine derivative targeting pancreatic adenocarcinoma. Ann Pancreat Cancer 2024;7:11.


