
Combination of axial and bivalve techniques in the grossing protocol for pancreaticoduodenectomy specimens in the assessment of ampullary and pancreatic adenocarcinomas and intraductal papillary mucinous neoplasia
Highlight box
Key findings
• The combination of the bivalve and axial techniques in the grossing protocol for pancreaticoduodenectomy (PD) specimens allows for a higher percentage of free margins (R0), taking into account the influence of the surgical technique, neoadjuvant therapy, and the expertise of the pathologist.
What is known and what is new?
• The axial technique allows for a good assessment of surgical margins with less distortion of the specimen and is sensitive in identifying R1 resections. However, it has the disadvantage of poorer identification of the tumor origin. In contrast, the bivalve technique provides a better evaluation of the tumor origin but compromises the assessment of margins and surfaces, which become distorted during bivalve.
• The execution of the bivalve technique, by making cuts below the posterior margin (PM) for opening the Biliary duct and through the super-anterior surface for opening the main pancreatic duct allows for less distortion of the PM while providing a good assessment of the intraductal tumor location.
• The use of this “dual protocol” is significantly associated with margin status, particularly in the case of intraductal papillary mucinous neoplasms (IPMNs). Additionally, the use of neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma has been associated with a reduction in tumor size, and both the medication received, the number of treatment cycles, and the surgical approach influence the final surgical margins.
What is the implication, and what should change now?
• The use of both techniques (bivalve and axial) in the grossing of PD specimens represents an option for pathologists, especially in cases where IPMN is suspected.
Introduction
Background
Pancreatic ductal adenocarcinoma (PDAC), the most common histological type of pancreatic malignancies (>90%), is associated with a poor prognosis. Only 10–20% of patients present with resectable tumors at diagnosis, and 70–90% experience recurrence within the first two years post-surgery (1). PDAC is the seventh leading cause of cancer-related deaths globally (2). While incidence rates vary across countries, global trends indicate a steady increase in PDAC diagnoses, rising by 0.5% annually since 2010. The mean age at diagnosis is 65 years, though an increasing incidence among younger patients has been observed (2,3).
Ampullary adenocarcinoma (AAC) is a malignant epithelial tumor of the small intestine, centered in the ampulla and exhibiting glandular differentiation. It represents 6–9% of all periampullary malignancies, with approximately 15% of pancreaticoduodenectomies (PDs) performed for AAC. AAC is more prevalent in men, with a median age of diagnosis around 60 years. Resected AAC has an overall 5-year survival rate of 45%. Prognostic factors include age, lymph node metastasis, perineural and vascular invasion, and margin status. Despite their small size, ampullary-ductal carcinomas are often aggressive (1).
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is an intraductal epithelial neoplasm composed of mucin-producing cells, originating in the main pancreatic duct or its branches. Gender distribution varies, with higher prevalence observed in Asian men and American women. The median age at diagnosis is approximately 65 years. Main duct-type IPMNs carry a higher risk of progression to invasive carcinoma. Five-year survival rates for resected IPMNs range from 85% to 100%, but decrease to 36%–90% when associated with PDAC (1).
In these neoplasms, resectability and staging are the primary factors influencing prognosis. Patients with negative margins (R0) have the most favorable outcomes (2). The definition of microscopic margin involvement (R1) has been a subject of extensive debate. Current evidence indicates that a margin clearance of ≥1 mm is associated with improved survival compared to <1 mm clearance and is a more reliable predictor of a true negative-margin resection. This concept has been endorsed by various scientific societies, including the Royal College of Pathologists (RCP) (4,5). When defining microscopic margin involvement (R1) as <1 mm clearance, it is critical to preserve the relationship between the inked margin and the underlying tissue. Achieving this requires thorough and extensive sampling (6).
One of the earliest reference studies on this topic, conducted by Esposito in 2008, reported a highly variable rate of microscopic margin involvement (R1), ranging from 16% to 85% (7). Since then, numerous studies have established correlations between margin involvement and both survival and tumor recurrence (1,8,9). The determination and precise definition of an “affected margin” has since become a cornerstone of pathological assessment.
Building on this premise, various grossing protocols have been developed. While their overarching principles are consistent, differences in terminology, the classification of certain surfaces as margins, and slicing techniques contribute to discrepancies in pathology reports among specialists. Furthermore, PD is a complex surgical procedure typically performed in tertiary care centers, where the handling of surgical specimens is restricted to specialized groups of pathologists.
Regarding terminology, the naming of margins varies significantly. For instance, Verbeke refers to a specific margin as the “superior mesenteric vein (SMV) surface” (10), while the Spanish Society of Pathology (SEAP) designates it as the “Medial or vascular resection margin (MRM)” (11), and the College of American Pathologists (CAP) identifies it as the “Uncinate margin” (12). Discrepancies are typically less pronounced for luminal and pancreatic transection margins (PTMs), as these are more easily identifiable.
Regarding the inclusion of certain surfaces as margins, particularly the anterior surface, the Japan Pancreas Society (JPS) recommends its consideration in grossing protocols, citing an increased risk of local recurrence when tumor cells are present (13). In contrast, the American Joint Committee on Cancer (AJCC) does not include this evaluation, as the anterior surface is not considered a true anatomical margin (14).
The slicing technique is another widely debated topic, as the method used directly impacts the evaluation of the specimen. Verbeke outlines two distinct techniques (10,15):
The bivalving technique involves probing the main pancreatic duct and common bile duct, followed by slicing the specimen along the plane of the probes to expose both ducts longitudinally. This approach enables precise evaluation of tumor infiltration and origin within the ducts. It is particularly beneficial for studying AAC, as it supports site-specific subclassification of ampullary tumors (1), and for diagnosing IPMN (6). However, its disadvantages include technical complexity and potential distortion of the surgical margins (15).
The axial technique involves making perpendicular cuts to the longitudinal axis of the duodenum. This method offers several advantages, including improved margin assessment and reduced specimen deformation. It is also reported to be more sensitive for identifying R1 resections (15). However, its main drawback is that the ampullary region often lies between sections, complicating accurate evaluation of tumor origin. This limitation is particularly relevant for distinguishing AAC and IPMN, as previously noted (6).
To the best of our knowledge, the only study comparing the axial and bivalve techniques for evaluating PD specimens is van Roessel’s [2021], which reported similar R1 rates (60%, P=0.71). Additionally, no significant difference was observed in the determination of tumor origin (P=0.21) (16). It is generally accepted that pathologists select the technique they consider most appropriate, based on the characteristics of the surgical specimen and the guidelines of their respective scientific societies.
Lymph node status is a crucial predictive factor for both short-term and long-term survival (≥5 years) (1). According to the RCP, the evaluation of at least 12 lymph nodes is required for an accurate diagnosis (5). The number of lymph nodes examined also serves as an indicator of the quality of the pathologist’s work (11). While anatomic division is not essential, individually submitted lymph nodes should be reported separately (6). Lymph node sampling must be performed “en bloc”, as “peeling” is discouraged due to its potential to compromise margin assessment (15,17).
Rationale, knowledge gap and study context
Due to the complexity of pancreatic surgery, it is typically performed in specialized centers, where evaluations are conducted by a limited number of pathologists. The extensive literature on the subject has resulted in widely varying terminology, often leading to challenges in report reproducibility.
In this scientific and academic context, our center was designated as a tertiary hospital specializing in hepatobiliary and pancreatic surgery in 2011. This designation brought together specialized pathologists, surgeons, and medical oncologists in the field. The growing number of patients referred from other hospitals and cities necessitated organizational restructuring and drove significant scientific advancements.
These developments prompted the implementation of a new grossing protocol for PD specimens. This “dual protocol” combined bivalve and axial techniques to accurately determine tumor origin or the presence of IPMN without compromising the evaluation of surgical margins. The dual protocol was introduced in January 2011 and has been consistently utilized in the pathology department of our hospital. Additionally, the “artery-first” surgical approach was adopted, and NAT was initiated, with the first patient treated in February 2012.
Objective
This study describes the dual protocol, its techniques and photographic documentation, aiming to evaluate its impact on the assessment of surgical margins and tumor location in PDAC, AAC, and IPMN. The analysis compares outcomes before [2004–2010] and after [2011–2024] its implementation, considering the influence of factors such as NAT and advancements in surgical techniques, particularly the “artery-first” approach. A key limitation of the axial method, as described, is the difficulty in identifying tumor origin—a challenge this study seeks to address.
Methods
Study design, sample selection and inclusion criteria
This descriptive, observational, and retrospective study includes all cases of PDs performed at Hospital Universitari i Politècnic La Fe, which is currently the regional reference center for pancreatic pathology (CSUR), between January 2004 and December 2024 for PDAC, AAC and IPMN. Eligible cases were those with a prior cytological diagnosis confirmed via fine-needle aspiration (FNA) in patients aged 18 years or older, of both sexes. The search was conducted using the pathology software PATWin LYS (Madrid, Spain, v.4.11.11.0), implemented in our center. All cases meeting these inclusion criteria were included in the study without imposing a maximum case limit. Case selection was conducted individually, without randomization or prior sample size calculation.
Exclusion criteria
Patients younger than 18 years, those who underwent distal pancreatectomies, and those undergoing PD for other neoplastic (e.g., cholangiocarcinoma) or non-neoplastic (e.g., pancreatitis, trauma) lesions were excluded.
Group formation
Subsequently, patients were divided into two groups: Group A and Group B. Group B included cases where the dual protocol was applied, while Group A comprised cases where it was not. Details of the protocol are outlined in the following section, “Implementation Steps of the Dual Protocol”. The selection process is illustrated in Figure 1.
- Group A: 33 cases evaluated between January 2004 and December 2010 by a group of 16 general pathologists with routine daily case assignments. Of these cases, 22 were assessed exclusively using the axial technique, and 11 were evaluated solely with the bivalve technique.
- Group B: consisted of 95 cases evaluated between January 2011 and December 2024 by two expert pathologists specializing in hepatobiliary and pancreatic pathology, following the implementation of the dual protocol.
Note: Given the differences in TNM classification from 2004 to 2021 (8th AJCC Protocol), as well as variations in nomenclature and margin involvement criteria (<1 mm), all cases were re-evaluated, with a primary focus on Group A. This review aimed to standardize nomenclature across all cases to ensure consistency and enable accurate statistical analysis.
Implementation steps of the dual protocol
The dual protocol was implemented between January and March 2011 by two expert pancreatic pathologists, both of whom remain part of the hospital staff and contributors to this study. The implementation was carried out in two distinct phases:
- Literature search (January–February 2011): the authors conducted a systematic review in PubMed, following PRISMA guidelines, and focused on publications from January 2004 to December 2010. The search utilized the keywords: “pancreaticoduodenectomy”, “grossing protocol for pancreaticoduodenectomy specimens”, “pancreatic adenocarcinoma prognosis”, “axial method for grossing pancreaticoduodenectomy specimens” and “bivalve method for grossing pancreaticoduodenectomy specimens”.
- Following the literature search, studies from the SEAP, the RCP and the AJCC, along with the works of Esposito and Verbeke referenced in the introduction, were selected. Based on the synthesized information, the protocol described in this article was developed.
- To achieve this, the following updates were incorporated: new definitions for free and involved margins, along with specific criteria for each margin; duct-opening techniques based on the bivalve method; slicing techniques derived from the axial method; recommendations for the number of tissue blocks for grossing; the minimum number of isolated lymph nodes to evaluate; neoadjuvant therapy (NAT), response grading scale; and the latest tumor-node-metastasis (TNM) staging system (8th AJCC System).
- Implementation (March–April 2011): the dual protocol, as outlined in this study, was implemented for all PD specimens received during this period. Additionally, the protocol was presented to the radiology and hepatobiliopancreatic surgery committees at our center.
Variables
The variables and their diagnostic criteria are detailed in Table 1. All variables were assessed during the study period (December 2024–January 2025), and no follow-up processes were conducted.
Table 1
| Variable | Values | Diagnostic criteria |
|---|---|---|
| Age | ≥18 years | Patients’ age at the time of surgery |
| Sex | Male/female | Self-explanatory |
| Status | Alive/deceased | Patients’ vital status as of the study date (December 2024) |
| Cause of death (as applicable) | Intraoperative or perioperative death/recurrence or metastasis/other causes unrelated to the study focus | In case of death, the relationship between the cause of death and PDAC/ampullary tumor/IPMN |
| “Artery-first” surgical approach | Yes/no | Self-explanatory |
| Neoadjuvant therapy | Yes/no | Self-explanatory |
| Type of neoadjuvant therapy | NCT/NCRT | Chemotherapy only (NCT)/chemotherapy + radiotherapy (NCRT) |
| NCT drug | FOLFIRINOX/gemcitabine plus nabpaclitaxel/gemcitabine plus abraxane/FOLFOXIRI/gemcitabine plus erlotinib/gemcitabine plus oxaliplatin | Self-explanatory |
| NCT cycles | 2 to 12 | Total number of cycles received by each patient |
| Tumor location | Ampulla/head | Self-explanatory |
| IPMN | Yes/no | Self-explanatory |
| IPMN location (as applicable) | Main pancreatic duct/accessory ducts | Self-explanatory |
| pT | pT1a: tumor less than or equal to 0.5 cm in greatest dimension; pT1b: tumor greater than 0.5 cm and less than 1 cm in greatest dimension; pT1c: tumor 1–2 cm in greatest dimension; pT2: tumor greater than 2 cm and less than or equal to 4 cm in greatest dimension; pT3: tumor greater than 4 cm in greatest dimension; pT4: tumor involves the celiac axis or the superior mesenteric artery, and/or common hepatic artery, regardless of size | “p”: pathological evaluation following the statutes of the 8th AJCC Protocol |
| ypT | Same as “pT” category | “yp”: pathological evaluation after neoadjuvant treatment following the statutes of the 8th AJCC Protocol |
| pN | pN0: no regional lymph node metastasis; pN1: metastasis in one to three regional lymph nodes; pN2: metastasis in four or more regional lymph nodes | Following the statutes of the 8th AJCC Protocol |
| ypN | Same as “pN” category | Following the statutes of the 8th AJCC Protocol |
| Histological response | 0 (no viable cancer cells); 1 [single cells or rare small groups of cancer cells (near complete response)]; 2 [residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells (partial response)]; 3 [extensive residual cancer with no evident tumor regression (poor or no response)] | Following the statutes of the Ryan Modified Score (TRS) |
| Pathologist expertise | Yes (pathologist specialized in pancreatic pathology)/no (general pathologist) | Specialization defined as >10 years of experience with multiple publications related to the specialty |
| Margin status | R0/R1 | R1 defined as <1 mm clearance |
| Affected margin (as applicable) | PM/MRM/PTM/luminal | Self-explanatory |
| Dual protocol | Yes/no (axial or bivalve method only) | Self-explanatory |
AJCC, American Joint Committee on Cancer; FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin; FOLFOXIRI, fluorouracil, leucovorin, oxaliplatin, and irinotecan; IPMN, intraductal papillary mucinous neoplasia; MRM, medial resection margin; NCT, neoadjuvant chemotherapy; NCRT, neoadjuvant chemotherapy and radiotherapy; PDAC, pancreatic adenocarcinoma; PM, posterior margin; PTM, pancreatic transection margin; TRS, Tumor Regression Score.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics, Version 22 (New York, USA). Categorical variables are presented as frequencies and percentages, while continuous variables are reported to two decimal places. Differences were analyzed using a two-tailed Chi-squared test, with an alpha level of 0.05. Statistical significance was defined as P≤0.05. No normality tests were applied. The analysis examined the statistical relationship between the use of the dual protocol and margin status, as well as the association between margin status and factors such as the use of NAT, the expertise of the pathologist, and the “artery-first” surgical approach.
Description of the dual protocol
Microscopic margin involvement (R1) was defined as a clearance of <1 mm. It is important to highlight that in our center, the use of frozen sections is limited to evaluating the PTM and peripancreatic lymph nodes during surgery. Intraoperative analysis is considered positive when a tumor or IPMN with high-grade dysplasia is identified, as this finding influences subsequent surgical procedures.
Pancreatic margins
- Luminal margins: these are the edges of the resection that are continuous with the lumen of the digestive tract (5,6,10,11,15):
- Proximal/gastric margin: located adjacent to the stomach;
- Distal/duodenal or intestinal margin: located adjacent to the duodenum or small intestine.
- Biliary margin: this margin is located adjacent to the bile duct (5,6,10,11,15).
- PTM: identified by the cross-section of pancreatic tissue and the main pancreatic duct (5,6,10,11,15).
- Circumferential margins: correspond to the anterior and posterior surfaces of the pancreas and include:
- Medial resection margin/vascular margin (MRM): the MRM surrounds the imprint of the SMV along its retroperitoneal course. It is identified by palpating and observing the characteristic groove or impression of the SMV, which appears curved and shiny. If a portion of the vein was resected during the procedure, the fragment will typically be found adhered to this margin (5,6,10,11,15).
- Posterior margin (PM): this margin represents the fibrous and rough surface located between the superior mesenteric artery (SMA)/SMV surface and the posterior duodenal wall. It is typically the most commonly involved margin in R1 specimens (15).
Note: This surface is a subject of debate. Some authors, such as Verbeke, propose dividing it into two distinct margins: the posterior surface and the SMA surface/uncinate margin (the rough surface medial and posterior to the SMV, dissected by the surgeon from the SMA) (15). In contrast, organizations like the SEAP consider both to constitute a single margin (11). This distinction is significant, as certain studies, such as van Roessel’s, have reported prognostic differences depending on which of these surfaces is involved (4).
In this study, both surfaces are considered a single margin in accordance with the guidelines of our national scientific society (SEAP). However, it is important to note that a complete photographic record and detailed notes are taken during the grossing process. As a result, the affected cases in this study specifically correspond to the “posterior” margin as defined by Verbeke, rather than the uncinate margin. Consequently, the findings align with the prognostic differences previously reported. - The anterior surface is not universally regarded as a true margin, with its classification varying depending on the concept adopted by each working group (15).
Dual protocol
- Specimen fixation: upon receipt in the Pathology Department, the duodenum is opened along its antimesenteric border to facilitate optimal fixation and minimize anatomical distortion of the ampullary region (Figure 2A). If the lesion is visible at this stage, a fresh sample should be taken for molecular studies to ensure better DNA preservation, enabling more reliable biomarker determination. This step is crucial for evaluating PD specimens, as recommended by the consensus of the SEAP and the Spanish Society of Medical Oncology (SEOM) (18). Specimens are fixed for approximately 24–48 hours before grossing, ensuring complete immersion in ample formalin for thorough fixation (5,6,10,11,15).
- Anatomy identification: the surgical specimen is carefully examined to familiarize the pathologist with its anatomical features (Figure 2B,2C) (5,6,10,11,15).
- Identification and margins staining: after fixation, the identification and staining of the previously described margins are performed (Figure 2D). Measurements of the specimen, including the duodenum, pancreas, and bile duct, should be recorded. Additionally, any sutures, stents, or surgical clips must be carefully removed (Figure 3A) (5,6,10,11,15).
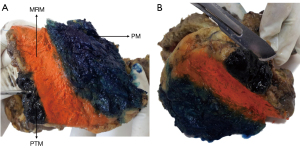 Figure 3 PD specimen. (A) Inking of margins. (B) Section of PTM. MRM, medial resection margin; PD, pancreaticoduodenectomy; PM, posterior margin; PTM, pancreatic transection margin.
Figure 3 PD specimen. (A) Inking of margins. (B) Section of PTM. MRM, medial resection margin; PD, pancreaticoduodenectomy; PM, posterior margin; PTM, pancreatic transection margin.- MRM: stained with Orange Ink.
- PTM: stained with Black Ink.
- PM: stained with Blue Ink.
- Sectioning of the PTM and luminal margins: after staining the PTM, a perpendicular cut to the lumen is made, producing a cylindrical fragment with one end stained with ink. Longitudinal cuts are then performed on this fragment to thoroughly examine the entire stained surface for potential tumor involvement (Figure 3B) (5,6,10,11,15).
- Specimen sectioning: the bile duct is cannulated using a thin, blunt-tipped probe inserted through the common bile duct, and its patency is assessed (Figure 4A,4B). The duct is then opened below the PM up to the ampulla to prevent deformation (Figure 4C). It is crucial to ensure that the incision for opening the duct is made below, rather than through, the PM—specifically between the anterior surface and the PM—to avoid incorrect assessment. Subsequently, the main pancreatic duct is cannulated, starting from the ampulla (Figure 5A,5B). If no resistance is encountered during cannulation, complete opening of the duct is not mandatory but is recommended. An incision is made with fine scissors along the superoanterior surface, which is not considered a true surgical margin, extending to the PTM (Figure 5C,5D). If resistance is encountered, it should not be forced (Figure 6A,6B). In such cases, the incision of the main duct should only extend to the point of resistance, following the superoanterior surface (Figure 6C,6D). It is essential to avoid contact with the PM. Subsequently, perpendicular cuts (4–5 mm thick) are made from the duodenum, enabling visualization of the tumor in relation to the margins, as dictated by the axial technique (Figures 7,85,6,10,11,15).
Note 1: Cannulation of the main pancreatic duct from the ampulla may inadvertently displace IPMN epithelium toward the transection margin of the main pancreatic duct, potentially leading to a misdiagnosis. This artifact can be identified by the presence of cells and mucinous material “floating” or “overlying” in the duct lumen or appearing as a “patch” on the epithelium. In contrast, a true IPMN would display intraductal proliferation of columnar mucin-producing cells, either flat or forming papillae, within the epithelium (1). Additionally, staining of the bile duct mucosa supports accurate histological determination, reducing the risk of misdiagnosis.
Note 2: It is important to emphasize that after the ducts are opened, a coronal cut exposing the anterior and posterior surfaces of the pancreas, typically performed in the bivalve technique, is not utilized. Instead, perpendicular cuts are made according to the axial technique. This approach combines the advantages of both methods while preserving the integrity of the margins, ensuring an uncompromised evaluation.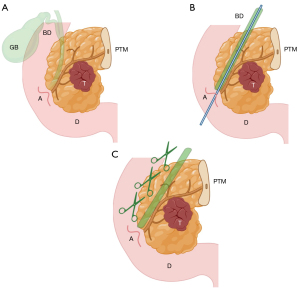 Figure 4 Biliary duct canalization and opening. (A) PD specimen and its anatomical references. (B) Cannulation of the biliary duct. (C) Opening of the biliary duct. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. A, ampulla; BD, biliary duct; D, duodenum; GB, gallbladder; PD, pancreaticoduodenectomy; PTM, pancreatic transection margin; T, tumor.
Figure 4 Biliary duct canalization and opening. (A) PD specimen and its anatomical references. (B) Cannulation of the biliary duct. (C) Opening of the biliary duct. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. A, ampulla; BD, biliary duct; D, duodenum; GB, gallbladder; PD, pancreaticoduodenectomy; PTM, pancreatic transection margin; T, tumor.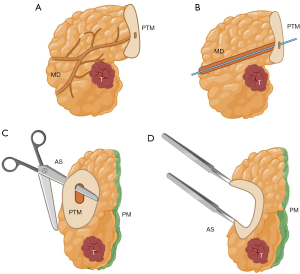 Figure 5 Main pancreatic duct opening (no resistance). (A) PD specimen and its anatomical references. (B) Cannulation of the main pancreatic duct without resistance. (C) If no resistance is encountered, opening the main pancreatic duct is recommended. This should be done along the superoanterior surface, avoiding contact with the posterior margin (stained with green ink). (D) Once opened, the duct is evaluated for lesions. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. AS, anterior surface; MD, main pancreatic duct; PD, pancreaticoduodenectomy; PM, posterior margin; PTM, pancreatic transection margin; T, tumor.
Figure 5 Main pancreatic duct opening (no resistance). (A) PD specimen and its anatomical references. (B) Cannulation of the main pancreatic duct without resistance. (C) If no resistance is encountered, opening the main pancreatic duct is recommended. This should be done along the superoanterior surface, avoiding contact with the posterior margin (stained with green ink). (D) Once opened, the duct is evaluated for lesions. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. AS, anterior surface; MD, main pancreatic duct; PD, pancreaticoduodenectomy; PM, posterior margin; PTM, pancreatic transection margin; T, tumor.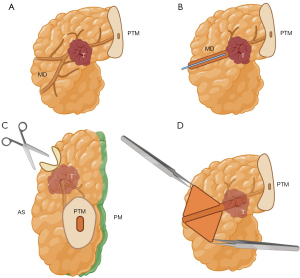 Figure 6 Main pancreatic duct opening (in case of obstruction). (A) PD specimen and its anatomical references. (B) Cannulation of the main pancreatic duct reveals obstruction of the duct by the tumor. (C) The duct should be opened along the superoanterior surface, avoiding contact with the posterior margin, up to the point of obstruction (stained with green ink). (D) Once opened, the duct is evaluated for lesions. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. AS, anterior surface; MD, main pancreatic duct; PD, pancreaticoduodenectomy; PM, posterior margin; PTM, pancreatic transection margin; T, tumor.
Figure 6 Main pancreatic duct opening (in case of obstruction). (A) PD specimen and its anatomical references. (B) Cannulation of the main pancreatic duct reveals obstruction of the duct by the tumor. (C) The duct should be opened along the superoanterior surface, avoiding contact with the posterior margin, up to the point of obstruction (stained with green ink). (D) Once opened, the duct is evaluated for lesions. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. AS, anterior surface; MD, main pancreatic duct; PD, pancreaticoduodenectomy; PM, posterior margin; PTM, pancreatic transection margin; T, tumor.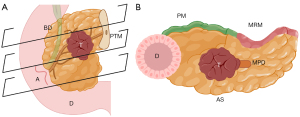 Figure 7 Axial cuts. (A) Schematic representation of axial cuts. (B) Schematic representation of an axial cut. The tumor can be seen at the center, in relation to the surgical margins and the duodenal lumen. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. BD, biliary duct; A, ampulla; D, duodenum; T, tumor; PTM, pancreatic transection margin; PM, posterior margin; MRM, medial resection margin; AS, anterior surface; MPD, main pancreatic duct.
Figure 7 Axial cuts. (A) Schematic representation of axial cuts. (B) Schematic representation of an axial cut. The tumor can be seen at the center, in relation to the surgical margins and the duodenal lumen. Created by Judith Gonzalez, MD, using the medical illustration software BioRender®. All images are accompanied by their respective publication licenses. Available online: https://BioRender.com/x62y584. BD, biliary duct; A, ampulla; D, duodenum; T, tumor; PTM, pancreatic transection margin; PM, posterior margin; MRM, medial resection margin; AS, anterior surface; MPD, main pancreatic duct.
Sampling
Sampling must be extensive, with a fragment containing only tumor tissue always obtained for subsequent molecular studies (Figure 9) (5,10,11,15,18).
- Luminal margins (gastric and duodenal): 2 blocks.
- Biliary margin and bile duct: transverse sections of the bile duct, with 1–2 tissue blocks.
- Complete sections of the tumor: the tumor bed should be entirely submitted, which is particularly critical for patients who have received NAT. A minimum of 5 tissue blocks is required.
- Tumor in relation to the ampulla of Vater: the tumor should be sampled “en bloc”, maintaining its contact with surrounding structures. At least 5 blocks.
- Tumor in relation to the MRM: a minimum of 5 tissue blocks should be obtained. The entire extent of the inked margin, from medial to lateral and proximal to caudal, must be included to ensure a thorough evaluation. This principle applies to all resection margins. For the MRM, in particular, the entire vascular groove should be submitted to allow for precise perpendicular measurement of the distance to the surrounding surfaces.
- Tumor in relation to the PM: at least 5 blocks.
- Tumor in relation to the PTM or RRM: at least 5 blocks.
- Lymph nodes: lymph nodes should be included en bloc alongside the specimen surface, with a minimum of 12 nodes submitted in 5–10 blocks, depending on the number of isolated nodes, while carefully noting their location. Care must be taken to avoid duplicating the count by mistakenly including both halves of a single node.
- Non-tumoral parenchyma (1–2 blocks).
- Additionally, any other observed lesions or areas of interest should be sampled.
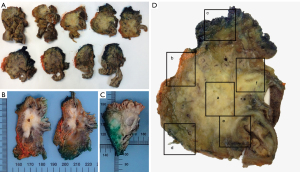
A minimum of 40–45 tissue blocks should be obtained per specimen, with additional blocks as needed depending on the specimen size, tumor dimensions, proximity to surgical margins, and the number of isolated lymph nodes.
Results
A total of 128 PD specimens were analyzed, comprising 73 men (57%) and 55 women (43%). The patients’ ages ranged from 31 to 91 years, with a mean age of 69.98 years. To date, 92 patients (71.9%) have died: 52 (56.52%) due to disease progression, 17 (18.48%) from postoperative complications, and the remaining 23 (25%) from causes unrelated to the disease. A total of 34 patients (26.6%) are still alive, while follow-up was lost for 2 patients (1.6%) who were not from our geographical area. The most common clinical presentation was monosymptomatic jaundice (54 cases, 42.2%), followed by constitutional syndrome (22 cases, 17.2%) and abdominal pain (22 cases, 17.2%). In 2 patients (0.8%), the diagnosis was made incidentally through radiological findings.
Twenty cases (15.6%) were classified AAC while the remaining 108 cases (84.4%) corresponded to PDAC. IPMN were identified in 17 cases (13.3%), with 14 (82.4%) involving the main pancreatic duct and 3 (17.6%) located in secondary ducts. Tumor differentiation was as follows: 46 cases (35.9%) were well-differentiated adenocarcinomas, 62 cases (48.4%) were moderately differentiated, and 20 cases (15.6%) were poorly differentiated. All specimens presented with a lesion, whether PDAC, AAC, or IPMN; no tumor-free specimens were identified.
Among the 113 cases that did not receive NAT, 5 cases (4.4%) were classified as pTis, 7 (6.2%) as pT1a, 9 (8%) as pT1b, 16 (14.2%) as pT1c, 47 (41.6%) as pT2, and 29 (25.7%) as pT3. In terms of nodal staging, 46 cases (40.7%) were pN0, 44 (38.9%) were pN1, and 23 (20.4%) were pN2. For the 16 cases that received NAT, 1 case (6.3%) was staged as ypT1a, 2 (12.5%) as ypT1b, 5 (31.3%) as ypT1c, and 8 (50%) as ypT2. Regarding nodal staging, 9 cases (56.3%) were ypN0, 6 (37.5%) were ypN1, and 1 (6.3%) was ypN2.
Group A consisted of 33 patients. Of these, 17 (51.5%) had free margins (R0), while 16 (48.5%) had involved margins (R1). Among the R1 cases, 14 (93.8%) had PM involvement, 1 (6.3%) had PTM involvement, and 1 (6.3%) had luminal margin involvement. Group B comprised 95 patients, 73 of whom (76.84%) had free margins. Among the remaining cases (R1; 16.1%), 13 (80%) had PM involvement, 1 (6.7%) had PTM involvement, 5 (33.3%) had MRM involvement, and 3 (20%) showed involvement of both the PM and MRM.
Among the 95 patients in Group B, 16 received NAT; 15 were treated with neoadjuvant chemotherapy (NCT; 93.8%) and 1 with neoadjuvant chemoradiotherapy (NCRT; 6.3%). NAT was administered exclusively in cases of PDAC, excluding AAC. Among these patients, 4 (25%) received FOLFIRINOX (fluorouracil, leucovorin, irinotecan, and oxaliplatin), 3 (18.8%) gemcitabine plus nab-paclitaxel, 2 (12.5%) gemcitabine plus Abraxane, 3 (18.8%) FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan), 1 (6.3%) gemcitabine plus erlotinib, 1 (6.3%) gemcitabine plus oxaliplatin, and 1 (6.3%) FOLFOXIRI plus stereotactic body radiation therapy (SBRT) 40 Gy. The treatment ranged from 2 to 12 cycles (1 patient discontinued after the second session due to poor tolerance), with an average of 5.13 cycles, a median of 4, and a standard deviation of 3.28.
The tumor response score (TRS) was assessed using the Modified Ryan Score (MRS) (19). Among the 16 patients, 1 (6.25%) achieved a complete pathological response (PCR), 4 (25%) demonstrated a good partial response (Ryan 1: single cells or rare small groups of cancer cells), and 11 (68.75%) exhibited a partial response (Ryan 2: residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells).
In Group A, 28 patients (84.8%) experienced recurrence within the first three years post-surgery, 2 (6.1%) died without evidence of recurrence, and 3 (9.1%) remain alive with no signs of recurrence. Among the 95 patients in Group B, 55 (57.3%) had recurrence within the first two years post-surgery, 24 (25.0%) died without evidence of recurrence, and 16 (16.7%) remain alive under medical follow-up.
The R0 rate in Group A was 51.5%, whereas it increased to 76.84% in Group B, representing a 25.3% improvement. The use of the dual protocol was significantly associated with surgical margin status (P=0.003), IPMNs (P=0.04), and tumor size determination (P=0.006). However, no significant relationship was found with nodal staging (P=0.22 for pN cases and P=0.13 for ypN cases) or type of carcinoma (ampullary vs. pancreatic; P=0.30). The dual protocol also showed a significant association with recurrence (P=0.04). Pathologist expertise was significantly linked to tumor size determination (P=0.003), the number of isolated lymph nodes (P=0.02), and surgical margin assessment (P=0.01).
NAT was significantly associated with tumor size reduction (P=0.003), but not with ypN stage (P=0.66) or margin status (P=0.88). However, the specific drug regimen administered was associated with margin status (P=0.02), as was the number of treatment cycles (P=0.03). Additionally, the surgical approach demonstrated a significant association with margin status (P=0.001). Table 2 summarizes the clinical and histopathological data of the patients regarding the dual protocol. Table 3 presents the study variables in relation to NAT, while Table 4 shows the chi-square test results for surgical margin status.
Table 2
| Clinical and histopathological data | Axial method or bivalve method only (n=33) | Dual protocol (bivalve + axial method) (n=95) | P value |
|---|---|---|---|
| Age (years) | 69.98 (mean) | 0.66 | |
| Sex | 0.94 | ||
| Male | 19 (57.58) | 54 (56.84) | |
| Female | 14 (42.42) | 41 (43.16) | |
| Status | 0.07 | ||
| Alive | 4 (12.12) | 30 (31.58) | |
| Deceased | 28 (84.84) | 64 (67.37) | |
| Unknown | 1 (3.03) | 1 (1.05) | |
| “Artery-first” surgical approach | 0.001 | ||
| Yes | 33 (100.00) | 5 (5.26) | |
| No | 0 | 90 (94.74) | |
| Adenocarcinoma | 0.30 | ||
| Ampullary | 7 (21.21) | 13 (13.68) | |
| Pancreatic | 26 (78.79) | 82 (86.32) | |
| IPMN | 0.04 | ||
| Present | 1 (3.03) | 16 (16.84) | |
| Absent | 32 (96.97) | 79 (83.16) | |
| pT | 0.006 | ||
| pTis | 4 (12.12) | 1 (1.05) | |
| pT1a | 3 (9.09) | 4 (4.21) | |
| pT1b | 5 (15.15) | 4 (4.21) | |
| pT1c | 8 (24.24) | 8 (8.42) | |
| pT2 | 9 (27.27) | 38 (40) | |
| pT3 | 4 (12.12) | 25 (26.32) | |
| pN | 0.22 | ||
| pN0 | 11 (33.33) | 35 (36.84) | |
| pN1 | 12 (36.36) | 32 (33.68) | |
| pN2 | 10 (30.30) | 13 (13.68) | |
| ypT | Not comparable | ||
| ypT1a | 0 | 1 (1.05) | |
| ypT1b | 0 | 2 (2.11) | |
| ypT1c | 0 | 5 (5.26) | |
| ypT2 | 0 | 8 (8.42) | |
| ypN | 0.13 | ||
| ypN0 | 0 | 9 (9.47) | |
| ypN1 | 0 | 6 (6.32) | |
| ypN2 | 0 | 1 (1.05) | |
| Margins | 0.003 | ||
| R0 | 17 (51.52) | 73 (76.84) | |
| R1 (PTM) | 1 (3.03) | 1 (1.05) | |
| R1 (PM) | 14 (56.85) | 12 (46.15) | |
| R1 (MRM) | 0 | 5 (5.26) | |
| R1 (luminal) | 1 (3.03) | 0 | |
| R1 (PM + MRM) | 0 | 3 (3.16) | |
| Pathologist expertise | 0.01 | ||
| Yes | 0 | 95 (100.00) | |
| No | 33 (100.00) | 0 | |
| NAT | 0.04 | ||
| Yes | 0 | 16 (16.84) | |
| No | 33 (100.00) | 79 (83.16) | |
| Histological response (Modified Ryan Score) | 0.09 | ||
| 0 | 0 | 1 (6.25) | |
| 1 | 0 | 4 (25.00) | |
| 2 | 0 | 11 (68.75) | |
| Recurrence | 0.04 | ||
| Yes | 28 (84.85) | 55 (57.89) | |
| No (died without evidence of recurrence) | 2 (6.06) | 24 (25.26) | |
| Alive without evidence of recurrence | 3 (9.09) | 16 (16.84) | |
Data are presented as n (%) unless otherwise specified. IPMN, intraductal papillary mucinous neoplasia; MRM, medial resection margin; NAT, neoadjuvant therapy; PM, posterior margin; PTM, pancreatic transection margin.
Table 3
| Clinical and histopathological data | Chemotherapy only (NCT) (n=15) | Chemotherapy + radiotherapy (NCRT) (n=1) | P value |
|---|---|---|---|
| Age (years) | 69.98 (mean) | 0.19 | |
| Sex | 0.43 | ||
| Male | 6 (40.00) | 1 (100.00) | |
| Female | 9 (60.00) | 0 | |
| Status | 0.37 | ||
| Alive | 5 (33.33) | 1 (100.00) | |
| Deceased | 10 (66.67) | 0 | |
| Unknown | 0 | 0 | |
| Adenocarcinoma | 0.30 | ||
| Pancreatic | 15 (100.00) | 1 (100.00) | |
| IPMN* | 0.87 | ||
| Present | 2 (13.33) | 0 | |
| Absent | 13 (86.67) | 1 (100.00) | |
| ypT | 0.003 | ||
| ypT1a | 0 | 1 (100.00) | |
| ypT1b | 2 (13.33) | ||
| ypT1c | 5 (33.33) | ||
| ypT2 | 8 (53.33) | ||
| ypN | 0.66 | ||
| ypN0 | 8 (53.33) | 1 (100.00) | |
| ypN1 | 6 (40.00) | ||
| ypN2 | 1 (6.67) | ||
| Margins | 0.88 | ||
| R0 | 12 (80.00) | 1 (100.00) | |
| R1 (PTM) | 2 (13.33) | 0 | |
| R1 (PM) | 0 | 0 | |
| R1 (MRM) | 0 | 0 | |
| R1 (luminal) | 0 | 0 | |
| R1 (PM + MRM) | 1 (6.67) | 0 | |
| Pathologist expertise | Not evaluable | ||
| Yes | 15 (100.00) | 1 (100.00) | |
| No | 0 | 0 | |
| Histological response (Modified Ryan Score) | 0.02 | ||
| 0 | 1 (6.67) | 0 | |
| 1 | 3 (20.00) | 1 (100.00) | |
| 2 | 11 (73.33) | 0 | |
| Recurrence | 0.68 | ||
| Yes | 10 (66.67) | 1 (100.00) | |
| No (died without evidence of recurrence) | 0 | 0 | |
| Alive without evidence of recurrence | 5 (33.33) | 0 | |
Data are presented as n (%) unless otherwise specified. *, a total of 17 patients were found to have IPMN in the surgical specimens; however, only 2 of these patients had received neoadjuvant therapy. IPMN, intraductal papillary mucinous neoplasia; MRM, medial resection margin; NCT, neoadjuvant chemotherapy; NCRT, neoadjuvant chemotherapy and radiotherapy; PM, posterior margin; PTM, pancreatic transection margin.
Table 4
| Variable | P (margin status) |
|---|---|
| Dual protocol | 0.003 |
| Pathologist expertise | 0.01 |
| Surgical approach (“artery-first”) | 0.001 |
| Neoadjuvant therapy | 0.88 |
| Drug received | 0.02 |
| Number of cycles received | 0.03 |
Discussion
Key findings and comparison with similar research
Changes in the percentage of involved margins in PD specimens following the implementation of a standardized grossing protocol have been reported in the literature, and R1 rates are often used as indicators of both treatment quality and the thoroughness of pathological evaluation.
Some studies have demonstrated an increase in R1 rates following protocol implementation. For example, Verbeke reported a rise from 53% to 85% using the axial technique (15). However, margin status should not be attributed solely to pathological examination. An elevated R0 rate may result from less rigorous pathological assessment of the margins or from improved treatments. In this regard, the evolution of surgical techniques (e.g., the artery-first approach) since the early 2000s, as well as the use of NAT, are both critical factors influencing these outcomes.
Regarding surgical techniques, Nguyen [2024] reported a 93.5% R0 rate using the “artery-first approach”, with the remaining 6.5% displaying PM involvement (20). Beginning in 2011, our surgical team expanded considerably, facilitating the implementation of the “artery-first” approach and increasing surgical volume. This factor proved significant in our study (P=0.001). Results are more variable when it comes to NAT. Palm [2023] found a 92% R0 rate (21), and Villano [2022] observed a higher frequency of R0 resections in patients receiving NAT compared to those undergoing upfront surgery (86.1% vs. 72%; P<0.001) (22). In contrast, Masuda [2024] detected no significant difference (23). van Veldhuisen [2023] documented an R0 status in 57.4% of patients, but the number of cycles was not associated with overall survival (OS) (24).
We observed a 25.3% increase in R0 resections following the implementation of the dual protocol, with a statistically significant association. Despite these advancements, the PM remains the most frequently involved margin, either alone or in conjunction with another margin. This finding persists even after employing more comprehensive grossing techniques and submitting a minimum of 40 tissue blocks, factors previously suggested to increase R1 rates (15).
When focusing solely on the pathological evaluation of margin status, the axial method is noted to be more sensitive for detecting R1 resections (15). Since the primary drawback of the bivalving method is potential disruption of the margins, our protocol entails opening the ducts below the PM to preserve the anterior surface and PM as intact as possible, a crucial step before proceeding with axial cuts. In addition to the influence of the surgical approach and NAT, as well as more extensive and meticulous sampling, we believe the increased R0 rate in our cohort may be attributed to complementing the axial method with a partial bivalve technique.
We identified a significant association between the combined use of both techniques and the diagnosis of IPMN, underscoring the advantages of the bivalving method in pinpointing this tumor origin. We propose that opening the duct allows for more reliable identification of IPMN, while perpendicular cuts to the duodenal wall preserve the tumor surface more effectively, thereby facilitating accurate tumor size determination.
Regarding AAC, our results did not show that combining axial and bivalve sectioning offers any advantage over the axial technique alone, consistent with Van Roessel’s findings (16). Moreover, the relationship with recurrence was significant, reaffirming that proper specimen evaluation directly affects patient prognosis, as previously described (1).
It is important, however, to note that obstruction of the main pancreatic duct, by preventing complete cannulation and thus full opening of the duct, hinders its thorough evaluation, becoming a limitation of the method.
About NAT, we recognize that residual fibrosis can make gross evaluation and precise tumor localization more challenging. Although we did not observe a significant association between NAT and either surgical margins or tumor recurrence, both the specific drug regimen and the number of treatment cycles were associated with these outcomes. Additionally, NAT was linked to a reduction in tumor size. It is important to note that these findings apply only to PDAC, as NAT is typically not indicated for AAC. Consequently, ampullary tumors depend heavily on pathological assessment, underscoring the importance of detailed photographic documentation and comprehensive sampling (total inclusion of the tumor bed) to correlate macroscopic and microscopic findings.
Another key factor is the pathologist’s expertise in evaluating PD specimens, as only a few pathologists typically perform these assessments. Greater experience generally translates into more accurate handling (16). In our cohort, pathologist expertise was significantly correlated with margin status, reinforcing this observation.
Strengths and limitations
Among the strengths of this study are its substantial sample size (128 PD) and the expertise of the pathology, medical oncology, and pancreatic surgery teams. However, several limitations should be noted, particularly in relation to technological advancements, evolving standards of care, and variations in pathologist training. Transitioning to a tertiary care center naturally entails the adoption of scientific and clinical innovations, which by themselves can enhance patient outcomes, making it challenging to attribute the increase in R0 margins solely to the dual protocol. Furthermore, the number of patients receiving NAT was small (16 cases), and although no significant association was observed, these findings do not exclude the potential relevance of NAT in larger cohorts.
Implications and actions needed
Our future objective is to further investigate the impact of NAT and other factors on both surgical margin involvement and long-term survival. We also aim to examine the mechanisms underlying therapeutic response, particularly molecular biology and genetic tumor alterations, in a larger cohort of patients.
Conclusions
Proper grossing of the surgical specimen is essential for accurate staging and determining patient prognosis, necessitating a standardized approach and the use of a common protocol. We hope that these findings will assist pathologists in their routine practice.
Acknowledgments
None.
Footnote
Data Sharing Statement: Available at https://apc.amegroups.com/article/view/10.21037/apc-24-26/dss
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-24-26/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-24-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics board of CEIM (Comité Ético de Investigación Medical Research Ethics Committee)-HOSPITAL UNIVERSITARIO Y POLITÈCNICO LA FE, No.: (CPMP/ICH/135/95) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Classification of Tumours Editorial Board. Digestive system tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2019 [cited 2024 august 8]. (WHO classification of tumours series, 5th ed.; vol. 1). Available online: https://tumourclassification.iarc.who.int/chapters/31
- Stoffel EM, Brand RE, Goggins M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023;164:752-65. [Crossref] [PubMed]
- Milella M, Bassi C, Boggi U, et al. Evolving pancreatic cancer treatment: From diagnosis to healthcare management. Crit Rev Oncol Hematol 2022;169:103571. [Crossref] [PubMed]
- van Roessel S, Kasumova GG, Tabatabaie O, et al. Pathological Margin Clearance and Survival After Pancreaticoduodenectomy in a US and European Pancreatic Center. Ann Surg Oncol 2018;25:1760-7. [Crossref] [PubMed]
- The Royal College of Pathologists. Standards and datasets for reporting cancers. Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of vater and common bile duct. The Royal College of Pathologists, London, 2013.
- Soer E, Brosens L, van de Vijver M, et al. Dilemmas for the pathologist in the oncologic assessment of pancreatoduodenectomy specimens : An overview of different grossing approaches and the relevance of the histopathological characteristics in the oncologic assessment of pancreatoduodenectomy specimens. Virchows Arch 2018;472:533-43. [Crossref] [PubMed]
- Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651-60. [Crossref] [PubMed]
- Osipov A, Nissen N, Rutgers J, et al. Redefining the Positive Margin in Pancreatic Cancer: Impact on Patterns of Failure, Long-Term Survival and Adjuvant Therapy. Ann Surg Oncol 2017;24:3674-82. [Crossref] [PubMed]
- McIntyre CA, Zambirinis CP, Pulvirenti A, et al. Detailed Analysis of Margin Positivity and the Site of Local Recurrence After Pancreaticoduodenectomy. Ann Surg Oncol 2021;28:539-49. [Crossref] [PubMed]
- Verbeke CS, Gladhaug IP. Dissection of Pancreatic Resection Specimens. Surg Pathol Clin 2016;9:523-38. [Crossref] [PubMed]
- Carmen Gómez-Mateo M, Sabater L, Ferrández-Izquierdo A. Protocolo de tallado, estudio e informe anatomopatológico de las piezas de duodenopancreatectomía cefálica por carcinoma de páncreas. Rev Esp Patol 2010;43:207-14. [Crossref]
- Washington K, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. College of American Pathologists; 2009.
- Japan Pancreas Society. Classification of Pancreatic Cancer. 2nd English ed. Tokyo: Kanehara; 2003.
- Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017.
- Campbell F, Verbeke C. Pathology of the pancreas: a practical approach. 2nd ed. Switzerland: Springer; 2021.
- van Roessel S, Soer EC, van Dieren S, et al. Axial slicing versus bivalving in the pathological examination of pancreatoduodenectomy specimens (APOLLO): a multicentre randomized controlled trial. HPB (Oxford) 2021;23:1349-59. [Crossref] [PubMed]
- Shi J, Basturk O. Whipple Grossing in the Era of New Staging: Should We Standardize? Diagnostics (Basel) 2019;9:132. [Crossref] [PubMed]
- Vera R, Ibarrola-de-Andrés C, Adeva J, et al. Expert consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology on the determination of biomarkers in pancreatic and biliary tract cancer. Clin Transl Oncol 2022;24:2107-19. [Crossref] [PubMed]
- Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141-6. [Crossref] [PubMed]
- Nguyen TK, Nguyen HH, Luong TH, et al. Pancreaticoduodenectomy with superior mesenteric artery first-approach combined total meso-pancreas excision for periampullary malignancies: A high-volume single-center experience with short-term outcomes. Ann Hepatobiliary Pancreat Surg 2024;28:59-69. [Crossref] [PubMed]
- Palm RF, Boyer E, Kim DW, et al. Neoadjuvant chemotherapy and stereotactic body radiation therapy for borderline resectable pancreas adenocarcinoma: influence of vascular margin status and type of chemotherapy. HPB (Oxford) 2023;25:1110-20. [Crossref] [PubMed]
- Villano AM, O'Halloran E, Goel N, et al. Total neoadjuvant therapy is associated with improved overall survival and pathologic response in pancreatic adenocarcinoma. J Surg Oncol 2022;126:502-12. [Crossref] [PubMed]
- Masuda H, Bhimani N, Chou A, et al. Pancreatic Body and Tail Adenocarcinoma: Upfront Resection Versus Neoadjuvant Therapy, A Contemporary Analysis. Pancreas 2024;53:e783-9. [Crossref] [PubMed]
- van Veldhuisen E, Klompmaker S, Janssen QP, et al. Surgical and Oncological Outcomes After Preoperative FOLFIRINOX Chemotherapy in Resected Pancreatic Cancer: An International Multicenter Cohort Study. Ann Surg Oncol 2023;30:1463-73. [Crossref] [PubMed]
Cite this article as: González-López J, Castells-Perona Ó, López-Valdivia C, Díaz-Beveridge R, Ballester-Ibáñez C, Montalvá-Orón E, López-Andújar R, Pérez-Rojas J. Combination of axial and bivalve techniques in the grossing protocol for pancreaticoduodenectomy specimens in the assessment of ampullary and pancreatic adenocarcinomas and intraductal papillary mucinous neoplasia. Ann Pancreat Cancer 2025;8:3.









