
Robotic single-site plus ONE-port distal pancreatectomy
Introduction
With the advance of laparoscopic techniques and instruments, laparoscopic pancreatectomy has become increasingly common. More specifically, laparoscopic distal pancreatectomy (DP) is regarded as an appropriate surgical option to treat benign and low-grade malignant lesions presenting in the left side of the pancreas. Although there are no randomized controlled studies comparing laparoscopic DP and open DP, an increasing number of case reports and literatures strongly suggest that the perioperative outcomes after laparoscopic DP are better than those following open DP, in terms of hospital stay duration and estimated intraoperative blood loss (1-5).
Recently, some expert surgeons tried to reduce the number of trocars in conventional laparoscopic surgery to enhance DP’s cosmetic and minimally invasive effects. Barbaros et al. (6) reported the first single-incision laparoscopic DP which was performed in a 59-year-old female to treat pancreatic metastasis from renal cell carcinoma. Since then, the number of cases treated with either laparoscopic single port (LSP) or laparoscopic reduced port (LRP) DP procedures has increased (7-13).
Despite the increasing number of LSP/LRP-DP and advances of laparoscopic instruments, fatigue and stress resulting from limited motion for instrument manipulation in the narrow surgical space (in current single port system) needs to be considered when performing LSP/LRP-DP. Therefore, in order to improve intraoperative surgical quality and reduce limitations, technical innovation is essential. In theory, robotic surgical systems can overcome limitations of laparoscopic surgery. This robotic technology is expected to work during performance of LSP/LRP-DP.
A robotic single-site surgical system has been known to facilitate laparoscopic single-port surgery (14-16). In addition, a stable, 3-D operation field can enhance surgeon’s ergonomic environment, and prevent the situation of right and left disorientation for triangular configuration during laparoscopic single-port surgery. It is believed that most intraoperative stress and fatigue result from the mechanics of laparoscopic single-port surgical system, such as fulcrum effect and limited motions of effector instrument. However, robotic surgical system automatically calculates the movement of surgeon’s console with the help of specially designed curved trocars and semi-flexible instruments, making it possible for the surgeon’s right and left hand to control the right- and left-sided screen instruments even if the instrument is attached to the left and right robotic arm, respectively.
If an additional robotic arm is used through another trocar in the abdomen, a wrist-like motion of instrument can be produced in the robotic single-site surgical system, which allows for a more effective reduced-port surgery. Considering there is no wrist like-motion in pure robotic single site robotic surgical system, technical advantages from additional port would be very helpful. Also, preoperative surgical rehearsal is another advantage of robotic surgery. Surgical techniques can be tested before they are applied directly to patients, which can enhance surgical quality and safety. Since October 2015, this author has been using our robotic single-site plus ONE port DP (RSS+1 DP) technique in selected cases (17).
Indication
Based on author’s experience, the best indications for RSS+1 DPS would be benign and low grade malignant tumors of the pancreas with the following conditions:
- Pancreatic tail tumor abutting splenic hilum, or involving spleen;
- Pathologic conditions that require less than 30% DP;
- No internal obesity; it was found that heavy omentum and redundant colon-splenic flexures derived from internal obesity made this surgical procedure very difficult and even impossible, as these factors concealed the main surgical field;
- Super-selected pancreatic cancer with above-mentioned tumor conditions; minimally invasive radical pancreatectomy in selected distal pancreatic cancer showed comparable oncologic outcomes in many clinical literatures. However, it should be reminded that margin-negative radical pancreatectomy is very important. Most pancreatic cancers in tail of the pancreas involving spleen or splenic hilum are usually large, and they can also invade surrounding organs, such as the spleen, stomach, and even colon mesentery. Since it may be very difficult to produce effective oncologic surgery by RSS+1 DPS, application of this procedure should be reconsidered, even for pancreatic cancer, and performed in only super-selected patients by highly experienced surgeons.
Single-port preparation (reverse-port technique)
Conventional commercialized port system (Figure 1A) will not be appropriate for RSS+1 DPS. According to original configuration, assist port site should be placed on the left side of the patient In our surgical technique, #② curved robotic arm is responsible for lifting the stomach, and this will narrow the space between #② external robotic arm and camera holding robotic arm, where assistant surgeon is supposed to be during surgical procedure. In this circumstance, the assistant surgeon cannot provide any help.
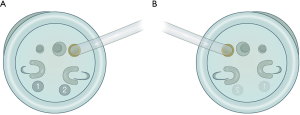
Therefore, RSS+1 DPS requires a specialized port system where the assist-port site is placed on the left side of patient, reverse-port (16) (Figure 1B). In fact, the author simply modified the original port-system for properly retracting the gallbladder toward upward lateral side to produce wide Calot triangle for safe cholecystectomy during RSS-cholecystectomy (16,18). When performing RSS+1 distal pancreatosplenectomy (DPS), patient’s left-sided assist-port placement makes some room for proper intervention by assistant surgeons during surgical procedure (Figure 2). Furthermore, it will be much easier for the assistant surgeon to change the robotic arm-instrument of additional ONE-port. Alternatively, currently available glove-port system (19,20) may be helpful in overcoming the disadvantages of conventional commercialized port system during RSS+1 DPS. Some of Korean robotic surgeons use it when performing this procedure.
Operation room setting
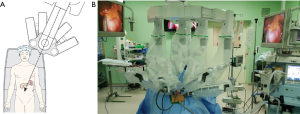
Alignment between patient and the patient-side cart of robotic surgical system is important. An imaginary line was appropriately drawn between the umbilicus and body of the pancreas, and the patient-side cart of robotic surgical system was moved to the patient table along this imaginary line (Figure 3A). According to current instructions, patient-side cart of the robotic surgical system is roughly supposed to approach the patient over his or her left-sided shoulder (Figure 3B). The other process for robot-docking is almost identical to that of usual robotic single site surgical procedure (15,18), except for an additional ONE-port site that must be considered by surgeons.
Placement of additional ONE-port
Placement of additional ONE-port is very important. Through this, surgeons can use effector instrument in wrist-like motion. Additional ONE-port should be a 12-mm conventional laparoscopic trocar (reason for this will be explained in the next section). In our early experiences, malposition of additional ONE-port resulted in severe external inter-arms collisions, especially between #① external robotic arm and #③ external robotic arm that is docked to the additional ONE port), leading to conversion to multiport robotic DP. In order to avoid extracorporeal inter-arms collisions, it would be helpful to find the appropriate site for additional ONE port using following steps (Figure 4).
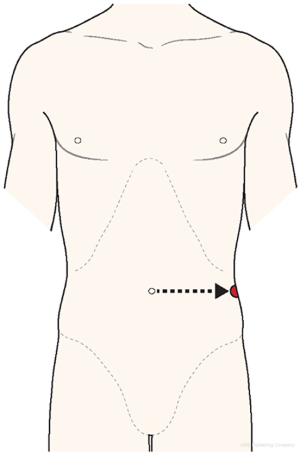
- Extend the imaginary line horizontally from the umbilicus to the left-sided flank.
- Palpate the left-sided flank area and identify the position just above descending colon-peritoneal flexure under camera scope vision.
- Place the 12-mm conventional trocar over that point.
Full lateral position of additional ONE-port will enhance the cosmetic effect in postoperative period. Lateral positioning of ONE-port would be barely seen from front-sided view of the patient.
Trocar in trocar technique
Introducing endo-GIA for dividing the pancreas through reverse-port is impossible, due to the size discordance between robotic assist-trocar and diameter of endo-GIA (10 vs. 12 mm). Therefore, endo-GIA should be applied through the additional port. For this purpose, placing robotic 8-mm trocar docked to the robotic surgical system into 12-mm conventional laparoscopic trocar is useful (Figure 5). During dissection of splenic vessels, an articulating robotic instrument can be used through this additional robotic 8-mm trocar in 12-mm conventional laparoscopic trocar. If necessary, endo-GIA can be introduced through 12-mm conventional laparoscopic trocar after temporarily removing robotic 8-mm trocar out of 12-mm trocar. This procedure can be simply performed by an assistant surgeon without difficulty; for this purpose, it would be ideal for assistant surgeon to be placed on the patient’s left side. This is another advantage of reverse-port system during RSS+1 DP.
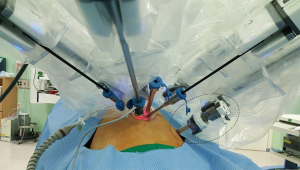
Of course, robotic endo-GIA (EndoWrist® Stapler) can also be used. Although this advanced technology can make the surgical procedure independent of an assistant surgeon’s skills, we found that surgeons cannot control the cutting speed of robotic endo-GIA for dividing the pancreas, and eventually leads to crushing of the pancreas rather than “dividing”. This phenomenon may be related to postoperative pancreatic fistula.
Surgical simulation
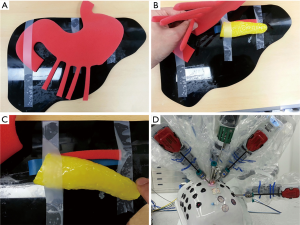
Before applying this procedure in clinical practice, a preoperative surgical rehearsal is recommended to help surgeons understand procedural concepts and to get used to new surgical environment for improving quality of surgery in actual performance. Since surgeons may encounter some technical issues during surgical simulation, they should prepare their own tactics to resolve potential problems that can arise during real clinical practice (Table 1, Figures 6 and 7).
Table 1
| Potential technical issues | Tactics in Yonsei |
|---|---|
| How to divide gastrocolic and gastrosplenic ligaments? | Use advanced robotic technology |
| Apply EndoWrist® vessel sealer | |
| How to lift stomach wall to expose distal part of the pancreas? | Use #② curved robotic arm to actively lift stomach wall |
| Use long-curved trocar to provide steady lifting power (sometimes) | |
| How to dissect splenic vessels? | Place EndoWrist® monopolar cautery instrument and EndoWrist® bipolar cautery instrument through additional port |
| Use intracorporeal tie and clip to ensure safe surgical procedure | |
| How to apply endo-GIA? | Use modified lasso technique* (21) to simplify surgical procedure |
| Train assist-surgeon on how to apply endo-GIA during surgical stimulation | |
| Consider using advanced robotic technology, but this may be inappropriate due to problem of pancreatic division speed | |
| Is it appropriate for the assist surgeon to manipulate near the patient’s side? | Test conventional commercialized single port system if it is appropriate for this surgical procedure |
*, modified lasso technique will be discussed later in this chapter. RSS, robotic single-site; DP, distal pancreatectomy.

Case and surgical technique
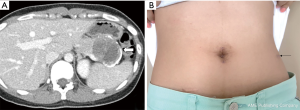
A 24-year-old female patient was admitted to hospital due to incidental finding of a mass in the pancreatic tail (Figure 8). All informed consents were given. Under the diagnostic impression of a solid pseudopapillary pancreatic neoplasm, she underwent robotic single-site plus ONE port DPS (Figure 9). Total operation time was 160 minutes, and the estimated intraoperative blood loss was less than 50 mL. When dissecting splenic vessels, angulating motion of surgical instrument through additional port made surgical procedure more effective and easy. Modified lasso technique was applied. No POPF was noted. Patient was discharged on the seventh postoperative day. Postoperatively, the wound appeared to be healing well (Figure 8). This case suggests that the main obstacles of LSP/LRP system, which includes surgical stress and ineffective instrument manipulation, can be resolved by using a robotic surgical system. More experience is required to determine the exact role of robotic single-site surgical system for performing LSP/LRP-DP.

Special considerations
Modified lasso technique (21)
Lasso technique was originally proposed by Velanovich (24) in 2006 for simple and effective laparoscopic DPS. It contains the following surgical procedures:
- Dissecting pancreas, splenic artery, and splenic vein altogether from the retroperitoneum;
- Encircling these structures altogether by the Penrose drain (“lasso”);
- Endo-GIA application to divide all of these structures at once.
Although the technique looks simple and effective, the original lasso technique harbors some potential risk of postoperative bleeding from the staple line in remaining splenic artery stump. We experienced a very similar potential complication after laparoscopic splenectomy (25). To prevent this potential safety issue, we always dissect splenic artery first and ligate it before applying lasso technique. Therefore, the pancreatic division line would be distal to splenic artery ligation site.
Spleen-preserving technique
Spleen-preserving procedure is both time and labor-consuming. In order to perform splenic vessel-conserving technique, small tributary vessels need to be controlled. In multi-port robotic surgical system, small metal-clips and wrist-like motion of instruments are very useful in this procedure, as they provide good surgical field. Our experiences have shown that multiport robotic surgical system is very useful in spleen-preserving DP (26-30). However, in RSS+1 system, ensuring surgical field may not be enough to perform spleen-preserving process, as the effector movement is not fully articulated except placing robotic instruments through additional ONE-port. Therefore, splenic vessel-conserving technique will not be effective by RSS+1 system. Both splenic vessel-sacrificing techniques may be acceptable in selected cases, but not all the time. Therefore, the best indication for RSS+1 DP would be pancreatic tail tumor involving splenic hilum or spleen which requires DP with splenectomy. Such indication would help prevent potential debates regarding the rationale on combined splenectomy in benign or low grade malignant tumor of the pancreas.
Acknowledgments
This study was supported by InSuk (Chi, Hoon Sang) Best Teacher Award (2015) of Severance Surgeon’s Alumni, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. The authors are also grateful to Dong-Su Jang (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea) for his help with the figures.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jin He) for the series “Robotic Surgery for Pancreatic Cancer” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.01.04). The series “Robotic Surgery for Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci 2013;20:421-8. [Crossref] [PubMed]
- Xie K, Zhu YP, Xu XW, et al. Laparoscopic distal pancreatectomy is as safe and feasible as open procedure: a meta-analysis. World J Gastroenterol 2012;18:1959-67. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- Sui CJ, Li B, Yang JM, et al. Laparoscopic versus open distal pancreatectomy: a meta-analysis. Asian J Surg 2012;35:1-8. [Crossref] [PubMed]
- Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc 2012;26:904-13. [Crossref] [PubMed]
- Barbaros U, Sumer A, Demirel T, et al. Single incision laparoscopic pancreas resection for pancreatic metastasis of renal cell carcinoma. JSLS 2010;14:566-70. [Crossref] [PubMed]
- Kim EY, You YK, Kim DG, et al. Dual-incision laparoscopic spleen-preserving distal pancreatectomy. Ann Surg Treat Res 2015;88:174-7. [Crossref] [PubMed]
- Machado MA, Surjan RC, Makdissi FF. Laparoscopic Distal Pancreatectomy Using Single-Port Platform: Technique, Safety, and Feasibility in a Clinical Case Series. J Laparoendosc Adv Surg Tech A 2015;25:581-5. [Crossref] [PubMed]
- Kuroki T, Adachi T, Okamoto T, et al. Single-incision laparoscopic distal pancreatectomy. Hepatogastroenterology 2011;58:1022-4. [PubMed]
- Misawa T, Ito R, Futagawa Y, et al. Single-incision laparoscopic distal pancreatectomy with or without splenic preservation: how we do it. Asian J Endosc Surg 2012;5:195-9. [Crossref] [PubMed]
- Haugvik SP, Rosok BI, Waage A, et al. Single-incision versus conventional laparoscopic distal pancreatectomy: a single-institution case-control study. Langenbecks Arch Surg 2013;398:1091-6. [Crossref] [PubMed]
- Han HJ, Yoon SY, Song TJ, et al. Single-port laparoscopic distal pancreatectomy: initial experience. J Laparoendosc Adv Surg Tech A 2014;24:858-63. [Crossref] [PubMed]
- Chang SK, Lomanto D, Mayasari M. Single-port laparoscopic spleen preserving distal pancreatectomy. Minim Invasive Surg 2012;2012:197429.
- Morelli L, Guadagni S, Di Franco G, et al. Da Vinci single site(c) surgical platform in clinical practice: a systematic review. Int J Med Robot 2016;12:724-34. [Crossref] [PubMed]
- Konstantinidis KM, Hirides P, Hirides S, et al. Cholecystectomy using a novel Single-Site((R)) robotic platform: early experience from 45 consecutive cases. Surg Endosc 2012;26:2687-94. [Crossref] [PubMed]
- Jung MJ, Lee SY, Lee SH, et al. Single-Site Robotic Cholecystectomy: Reverse-Port Technique. Medicine (Baltimore) 2015;94:e1871 [Crossref] [PubMed]
- Kim SH, Kang CM, Lee WJ. Robotic single-site plus ONE port distal pancreatectomy. Surg Endosc 2017;31:4258-9. [Crossref] [PubMed]
- Lee SH, Jung MJ, Hwang HK, et al. The first experiences of robotic single-site cholecystectomy in Asia: a potential way to expand minimally-invasive single-site surgery? Yonsei Med J 2015;56:189-95. [Crossref] [PubMed]
- Ko JW, Lee JW, Kwon SW, et al. Advantages of the glove port docking technique in robotic single-site cholecystectomy: comparison with the conventional silicone port. J Robot Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Lee H, Lee DH, Kim H, et al. Single-incision robotic cholecystectomy: A special emphasis on utilization of transparent glove ports to overcome limitations of single-site port. Int J Med Robot 2017;13: [Crossref] [PubMed]
- Kawasaki Y, Hwang HK, Kang CM, et al. Improved perioperative outcomes of laparoscopic distal pancreatosplenectomy: modified lasso technique. ANZ J Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Kang CM. Preoperative surgical simulation for robotic single site plus ONE-port DPS. Asvide 2018;5:097. Available online: http://asvidett.amegroups.com/article/view/22992
- Kang CM. Robotic single site plus ONE-port DPS for young female patient with solid pseudopapillary tumor of the pancreas. Asvide 2018;5:098. Available online: http://asvidett.amegroups.com/article/view/22993
- Velanovich V. The lasso technique for laparoscopic distal pancreatectomy. Surg Endosc 2006;20:1766-71. [Crossref] [PubMed]
- Choi SH, Kang CM, Hwang HK, et al. Reappraisal of anterior approach to laparoscopic splenectomy: technical feasibility and its clinical application. Surg Laparosc Endosc Percutan Tech 2011;21:353-7. [Crossref] [PubMed]
- Choi SH, Kang CM, Lee WJ, et al. Robot-assisted spleen-preserving laparoscopic distal pancreatectomy. Ann Surg Oncol 2011;18:3623. [Crossref] [PubMed]
- Kim DH, Kang CM, Lee WJ, et al. The first experience of robot assisted spleen-preserving laparoscopic distal pancreatectomy in Korea. Yonsei Med J 2011;52:539-42. [Crossref] [PubMed]
- Hwang HK, Chung YE, Kim KA, et al. Revisiting vascular patency after spleen-preserving laparoscopic distal pancreatectomy with conservation of splenic vessels. Surg Endosc 2012;26:1765-71. [Crossref] [PubMed]
- Hwang HK, Kang CM, Chung YE, et al. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc 2013;27:774-81. [Crossref] [PubMed]
- Lee LS, Hwang HK, Kang CM, et al. Minimally Invasive Approach for Spleen-Preserving Distal Pancreatectomy: a Comparative Analysis of Postoperative Complication Between Splenic Vessel Conserving and Warshaw's Technique. J Gastrointest Surg 2016;20:1464-70. [Crossref] [PubMed]
Cite this article as: Kang CM. Robotic single-site plus ONE-port distal pancreatectomy. Ann Pancreat Cancer 2018;1:12.

